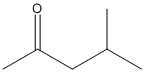
first of all, there's a big difference between almost the same and the same. The first thing you want to do for question 137 is draw out 4-methyl-2-pentanone:
(you'll see that this is correct because the longest carbon chain is 5 carbons, the carbonyl is in the 2 position, and the methyl group is in the 4 position).
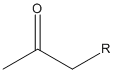
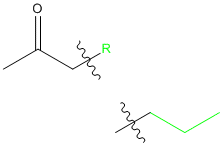
Now, in Scheme 1, they show the malonic ester synthesis, but the acetoacetic ester synthesis is exactly the same, except that instead of having a carboxylic acid as the product, for the acetoacetic ester synthesis, the product is a ketone. The product of the acetoacetic ester synthesis should look like this:
In both cases, the R is from the RX that we use in the alkylation step.
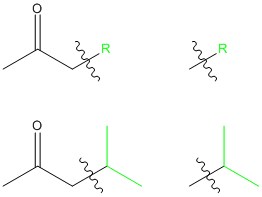
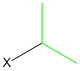
So, to figure out what alkyl halide we need to use, we simply have to look at the product we want and see what the correct R group is:
So, based on this, the appropriate R group must be:
(the little squiggly line means that the bond that it crosses is not part of the R group, but is where the R group will connect to the halide)
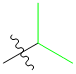
Thus, the appropriate alkyl halide is
This is an isopropyl halide, so the correct choice is isopropyl iodide.
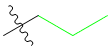
Now, let's see what would happen if I used propyl chloride:
The part in green is the R group:
Remember the form of the product so we'll know where the R group goes:
Thus:
Which gives us 2-hexanone as our product:
Questions on the MCAT that introduce new reactions are almost always trying to test whether you can recognize the pattern and follow it. They will usually give you a figure or something that has a general form of the product, and then give you new questions that require you to simply follow that general form.
Question 140 can be done in 2 ways. You can either recognize A as the correct answer immediately, or you can eliminate the other 3 answer choices. Let's eliminate first, then explain why A is right.
We can eliminate B because it doesn't make sense. B says that the proton on the alpha carbon is more acidic the second time, so a weaker base pulls off a more strongly acidic proton more effectively. But that's just silly. A weaker base isn't more effective at pulling off a strongly acidic proton. A strong base will pull off the proton just as effectively, if not more effectively, than a weaker base. There would be no point in changing bases to make it weaker. It's like saying that a bodybuilder will have more trouble picking up a 5 pound weight than an 8-year-old would. Sure, when the weight is only 5 lbs, the 8 year old can probably pick it up. But that doesn't mean the bodybuilder wouldn't be able to pick it up just as easily. So B is definitely incorrect.
C is also definitely incorrect. C says that, because the monoalkylated product is more sterically hindered, you'll need a larger base. But it's just the opposite. If steric hindrance is a problem, you use a smaller base so that it can find its way through the crowded space.
D can be eliminated for a few reasons. First of all, it's just not true. Ethanol is relatively polar, so adding an alkyl group to the molecule you want to dissolve in ethanol will make it less polar and therefore less soluble in methanol. Also, the answer choice claims that, since the monoalkylated product is more soluble in ethanol, that means that you have to change solvents. But if you think about it, why would increased solubility mean you'd have to change solvents. If you were doing the initial alkylation in ethanol, and your product becomes more soluble in ethanol, you'd probably want to keep it in ethanol. If you weren't doing the initial alkylation in ethanol, then why do you care about the ethanol solubility?
So that leaves choice A. Choice A is the opposite of choice B, and makes sense. It says that you change to a stronger base because the first alkylation made the alpha proton less acidic. Logically, this makes sense since, assuming that their statement that the alpha proton is less acidic is true, your original weaker base may not have been strong enough to pull off the less acidic proton, so now you need a stronger one to pull off the more weakly acidic proton. The other reason we can be sure of our answer is because the first part of the statement is true. Alkyl groups are electron donating. Since deprotonation of the alpha carbon leaves us with a carbanion, electron donating groups decrease the stability of the carbanion. This means that the alpha carbon is less acidic with the alkyl group because the conjugate base that you get after deprotonation is less stable.
If you notice, each answer choice was in the form:
"Because blah blah is true, blah blah is needed."
However, to answer this question, you never actually had to know if the "because blah blah is true" part was actually true. All you had to recognize was that the "blah blah is needed" part was not a logical conclusion that could be drawn from the "because blah blah is true" part.
Hope that helps



 ) lab procedure that is used to verify the concentration of the titrant.
) lab procedure that is used to verify the concentration of the titrant.