This one is Passage Set 2 of Chemical and Physical Foundations. It is number 9. The question asks what are the units for the rate constant ku? I understand how they calculated the units. I just don't understand the concept and what they are talking about! I don't know how to make sense of the order of reaction either. Thank you in advance!
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
AAMC Guide Practice Problem
- Thread starter RH8448
- Start date
- Joined
- Jul 1, 2015
- Messages
- 15
- Reaction score
- 1
I am also confused on what the background knowledge required to answer this question is...anyone? The question is what are the units of the rate constant (k sub-u) for protein unfolding and the answer is 1/seconds.
- Joined
- Jul 1, 2015
- Messages
- 15
- Reaction score
- 1
I think I figured out what the answer key is trying to say. Check out the Khan Academy video (Chemical Processes->Kinetics->Elementary Rate Laws) for more info but basically any elementary reaction (one-step) is the only kind where you can take the coefficient and use it as a superscript in the rate law. So the rate law for the protein unfolding would be: rate = k_u [protein]^1. Since rate has to be M/s, the k_u has units of 1/s. I hope that's right but if not hopefully someone will point out my mistake.
Last edited:
- Joined
- Jul 14, 2015
- Messages
- 16
- Reaction score
- 35
I am unclear on how it was known that the protein unfolds by a unimolecular mechanism. Was it given by the equation k_u = A*e^(-deltaG*/k_bT) or should we know that proteins engage in unimolecular reactions?
- Joined
- Jun 20, 2016
- Messages
- 264
- Reaction score
- 48
This is something that you just have to memorize for the exam.
You will notice that the equation given in the passage is the same exact equation for 1st order reactions, so you would want to use first order unit rate constant, which in this case is s^-1.
For 3rd order it is (1/M^2*s)
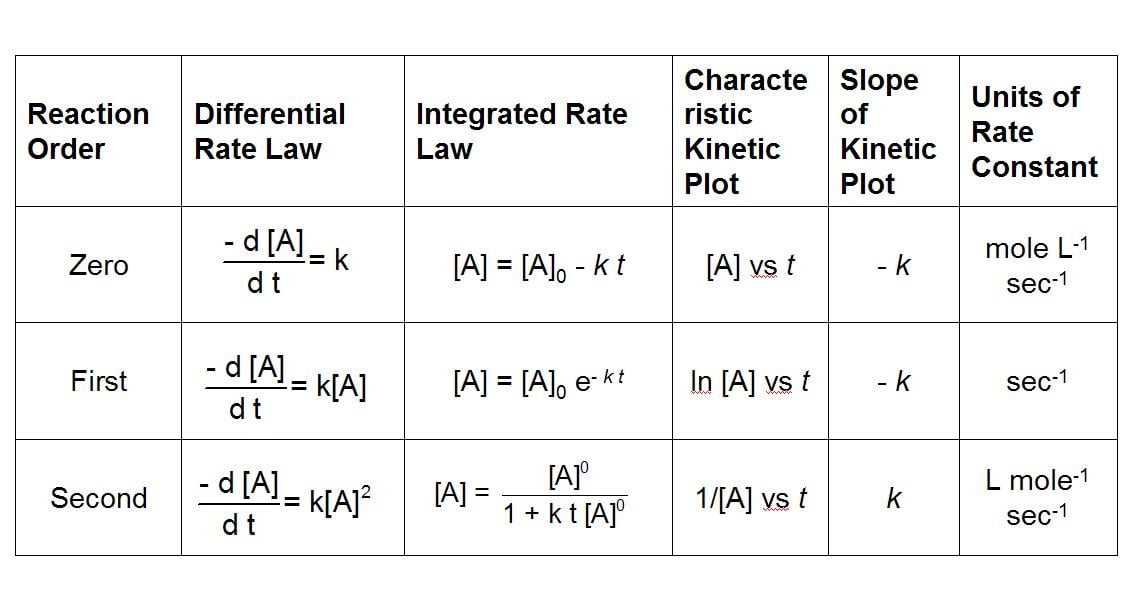
You will notice that the equation given in the passage is the same exact equation for 1st order reactions, so you would want to use first order unit rate constant, which in this case is s^-1.
For 3rd order it is (1/M^2*s)
I don't know the specific question, but if it's referring to a step of protein folding which can then be approximated as an elementary step, then it must be first-order since it can only depend directly on protein concentration. That also gives you a rate constant unit of 1/s. Remember that for all elementary steps and only for elementary steps, the exponent on the rate law is equal to the stoichiometry of the reaction. So in this case, the reaction would be F1 ---> F2, where F1 is fold at time 1 and F2 is the subsequent fold at time 2. Thus, the rate law would be k*[F1]^1.
- Joined
- Jul 30, 2016
- Messages
- 177
- Reaction score
- 39
I don't know the specific question, but if it's referring to a step of protein folding which can then be approximated as an elementary step, then it must be first-order since it can only depend directly on protein concentration. That also gives you a rate constant unit of 1/s. Remember that for all elementary steps and only for elementary steps, the exponent on the rate law is equal to the stoichiometry of the reaction. So in this case, the reaction would be F1 ---> F2, where F1 is fold at time 1 and F2 is the subsequent fold at time 2. Thus, the rate law would be k*[F1]^1.
This is something that you just have to memorize for the exam.
You will notice that the equation given in the passage is the same exact equation for 1st order reactions, so you would want to use first order unit rate constant, which in this case is s^-1.
For 3rd order it is (1/M^2*s)

yeah, all I used for this Q was my knowledge shown in the table. Protein unfolding, with no other reactant mentioned PLUS they give you the equation right in the passage.
Fairly easy (IMO) to see it must be 1st order. Don't overthink it, its just the AAMC, so repetitive in their questions, I love it.
- Joined
- Aug 30, 2016
- Messages
- 2
- Reaction score
- 0
I can't say about this issue.
- Joined
- May 20, 2014
- Messages
- 118
- Reaction score
- 21
what would be the hints for second order? would it be protein and another reactant involved in unfolding? That equation they give is only relevant for 1st order right?yeah, all I used for this Q was my knowledge shown in the table. Protein unfolding, with no other reactant mentioned PLUS they give you the equation right in the passage.
Fairly easy (IMO) to see it must be 1st order. Don't overthink it, its just the AAMC, so repetitive in their questions, I love it.
what would be the hints for second order? would it be protein and another reactant involved in unfolding? That equation they give is only relevant for 1st order right?
Yeah, it becomes more complicated as you have more reactants floating around, but if you know that in the elementary steps, there are two reactants involved (say the protein that is being folded and a chaperone protein) then it would be second-order.
Similar threads
- Replies
- 3
- Views
- 2K
- Question
- Replies
- 1
- Views
- 4K
