7
752779
Hey guys I was watching Khan Academy, when they were calculating the pH of a strong acid: they were using pH= -log [H3O+] to calculate pH for the strong acid.
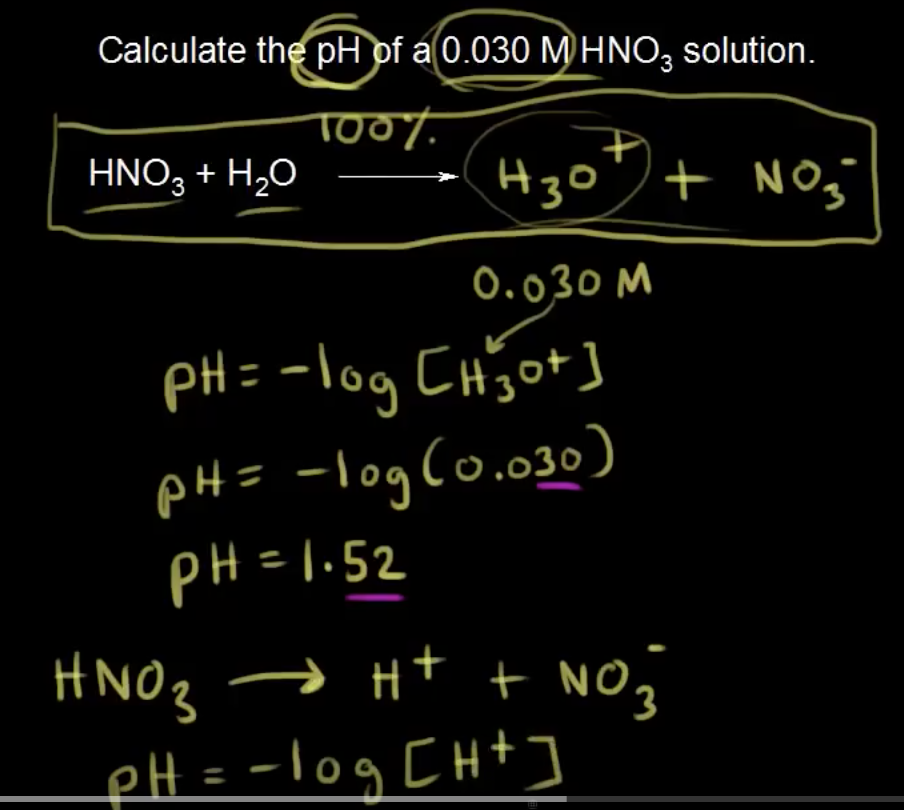
And here they used a different way to solve for pH in a weak acid. They were going to use the quadratic equation, but they have shorten it. However what I am confused is, these two questions seems to be similar, both asking to solve for pH of a solution providing concentration, but they used different method to solve for it. Is strong acid solve differently than weak acid?
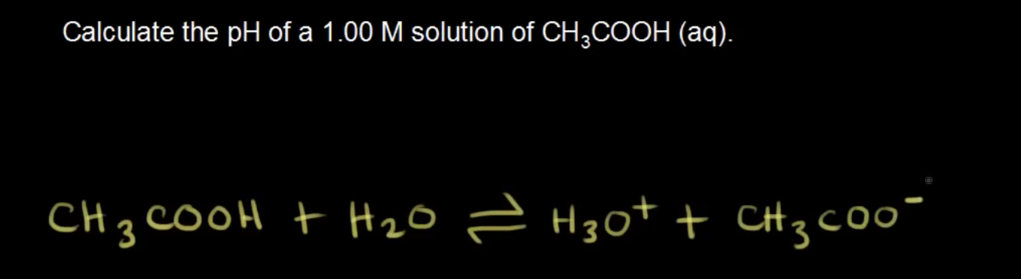
And here they used a different way to solve for pH in a weak acid. They were going to use the quadratic equation, but they have shorten it. However what I am confused is, these two questions seems to be similar, both asking to solve for pH of a solution providing concentration, but they used different method to solve for it. Is strong acid solve differently than weak acid?
