- Joined
- Jul 25, 2008
- Messages
- 67
- Reaction score
- 1
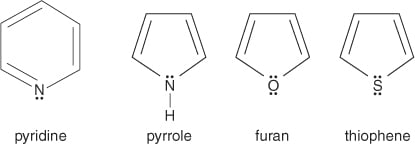
I still don't understand what the difference between the anti and non aromatic are. anti-aromatic is 4n? but there are situations where 4n can be non-aromatic as well. Someone please help with this, the other posts did not help much.
Thanks
Thanks