- Joined
- Jan 20, 2008
- Messages
- 1,270
- Reaction score
- 19
I saw this on a practice test and couldn't really get my head around it. Here is the structure being considered:
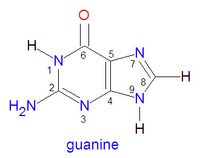
The question asks which nitrogen is most basic out of 2, 1, 7, or 9. I thought that the bond with more s-character increased in acidity, but the answer is supposed to be 7. I understand that the lone pairs of the Nitrogens on the left ring are tied up in resonance and on the right are tied up in aromaticity, but I still don't see how you can factor all that in to answer the question.
Can someone enlighten me? Thank you in advance.

The question asks which nitrogen is most basic out of 2, 1, 7, or 9. I thought that the bond with more s-character increased in acidity, but the answer is supposed to be 7. I understand that the lone pairs of the Nitrogens on the left ring are tied up in resonance and on the right are tied up in aromaticity, but I still don't see how you can factor all that in to answer the question.
Can someone enlighten me? Thank you in advance.
