- 28. Which is the best resonance structure for the following molecule?
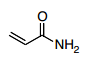
- A.
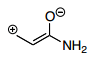
- B.
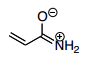
- C.
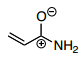
- D.
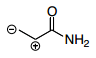
- E.
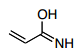
Answer is B - A.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
dat bootcamp orgo question
- Thread starter Tmac111
- Start date
- Joined
- Jul 8, 2014
- Messages
- 23
- Reaction score
- 6
Answer B would be more stable since it doesn't violate the octet rule which is more important than electronegativity.
- Joined
- Dec 2, 2012
- Messages
- 2,359
- Reaction score
- 1,046
Answer B would be more stable since it doesn't violate the octet rule which is more important than electronegativity.
Does have anything to do with
CARDIO?
- Joined
- Jul 8, 2014
- Messages
- 23
- Reaction score
- 6
what's CARDIO?
- Joined
- Dec 2, 2012
- Messages
- 2,359
- Reaction score
- 1,046
CARDIO
http://www.masterorganicchemistry.com/2010/09/22/five-key-factors-that-influence-acidity/
I just read it.
I think I was mistaken....
http://www.masterorganicchemistry.com/2010/09/22/five-key-factors-that-influence-acidity/
I just read it.
I think I was mistaken....
- Joined
- Dec 2, 2012
- Messages
- 2,359
- Reaction score
- 1,046
Do you have any tips for resonance?
- Joined
- Jul 8, 2014
- Messages
- 23
- Reaction score
- 6
I just know that the octet rule is the most important one and conjugated pi systems count for a more stable molecule and then the electronegativity of the atom.
- Joined
- Jul 8, 2014
- Messages
- 23
- Reaction score
- 6
I think this is right because my orgo professor taught us that but I totally forgot it, thanks for reminding meCARDIO
http://www.masterorganicchemistry.com/2010/09/22/five-key-factors-that-influence-acidity/
I just read it.
I think I was mistaken....
Btw do you have any tips for QR and RC?? I'm really struggling with these sections
- Joined
- Dec 2, 2012
- Messages
- 2,359
- Reaction score
- 1,046
RC:
I read quickly through each paragraph, 1 by 1 , and during or after each read paragraph I jot down key words or topic from that particular paragraph.
Give each paragraph a numerical position ex: paragraph 1, paragraph 2, etc.
Qr: do problems sets of 10 take a break and review. And repeat.
I read quickly through each paragraph, 1 by 1 , and during or after each read paragraph I jot down key words or topic from that particular paragraph.
Give each paragraph a numerical position ex: paragraph 1, paragraph 2, etc.
Qr: do problems sets of 10 take a break and review. And repeat.
- Joined
- Jul 8, 2014
- Messages
- 23
- Reaction score
- 6
Thank youRC:
I read quickly through each paragraph, 1 by 1 , and during or after each read paragraph I jot down key words or topic from that particular paragraph.
Give each paragraph a numerical position ex: paragraph 1, paragraph 2, etc.
Qr: do problems sets of 10 take a break and review. And repeat.
- Joined
- Dec 2, 2012
- Messages
- 2,359
- Reaction score
- 1,046
Sharing is caring
The thing I don't get about this, is the negative charge should be on the most electronegative atom, the oxygen, and the positive charge should be on the least electronegative, the carbon, right? Answer choice B has the positive charge on a electronegative atom? I thought the answer should be C
- 28. Which is the best resonance structure for the following molecule?

Incorrect
- A.

- B.

- C.

- D.

- E.

Answer is B
If you have Chad's Videos you should watch those; he explains it very well. I forgot some but this is some simple rules/explanations of mine.
1) you want least amount of charge
2) if there is charges put the negative charge on most electronegative atom (oxygen)
3) try to maintain the octet rule for all atoms
A) Oxygen has negative charge but C has positive charge. C doesn't really want to be positive; better to have it on N
B) C atoms looks good and see explanation for A
C) again C doesn't work well w/ positive charge
D) see above
E) no shifting/rearranging H's
Similar threads
- Replies
- 1
- Views
- 624
