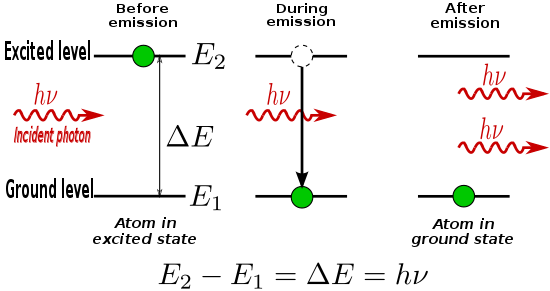
Suppose an electron goes from a second energy orbital to the first energy orbital, which is equal to the energy difference between the two energy orbitals. Then why is it that we subtract the second energy orbital from the first energy orbital? How can the second energy orbital possibly be less energy?
Dropping an Energy Level and Photon Emission
- Thread starter Yuppie202
- Start date