So I thought that the major determinants of product formation for E2 were:
1) LG and Hydrogen to be deprotonated (taken by the base) must be anti
2) Once that was fulfilled you went for the most stable product - more substituted Alkene product is more stable.
But a question I had said that if the base is bulky, but strong, it will attack the least substitued carbon forming the Hoffman Product.
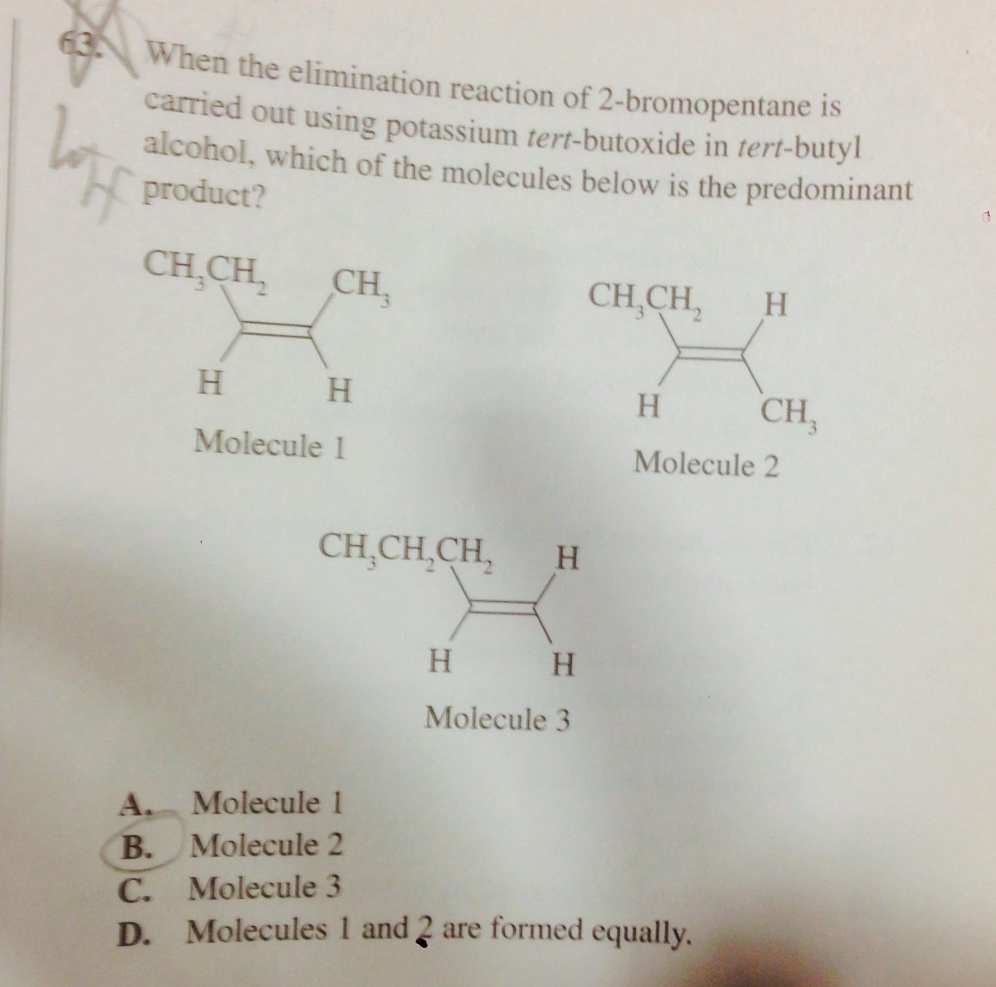
So the answer here is C apparently. Can someone clarify when bulkiness of the base takes precedence over forming the most stable product? here thats the difference between forming a more substitued alkene product (# 2 vs #3)
1) LG and Hydrogen to be deprotonated (taken by the base) must be anti
2) Once that was fulfilled you went for the most stable product - more substituted Alkene product is more stable.
But a question I had said that if the base is bulky, but strong, it will attack the least substitued carbon forming the Hoffman Product.
So the answer here is C apparently. Can someone clarify when bulkiness of the base takes precedence over forming the most stable product? here thats the difference between forming a more substitued alkene product (# 2 vs #3)
