- Joined
- Feb 16, 2016
- Messages
- 594
- Reaction score
- 96
Approximately what is the net work done on the gas as it goes directly from state A to state B?
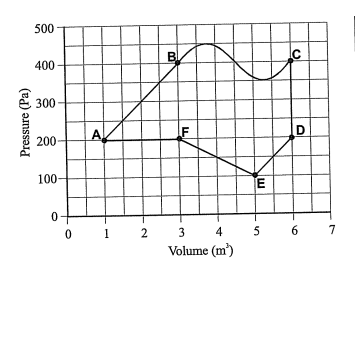
Can someone explain the steps needed to solve this problem?
I understand from A to B is an expansion because the work is being done by the gas. Work should be +, right? But it is asking for work done ON the gas? Lost. Help.
Can someone explain the steps needed to solve this problem?
I understand from A to B is an expansion because the work is being done by the gas. Work should be +, right? But it is asking for work done ON the gas? Lost. Help.
