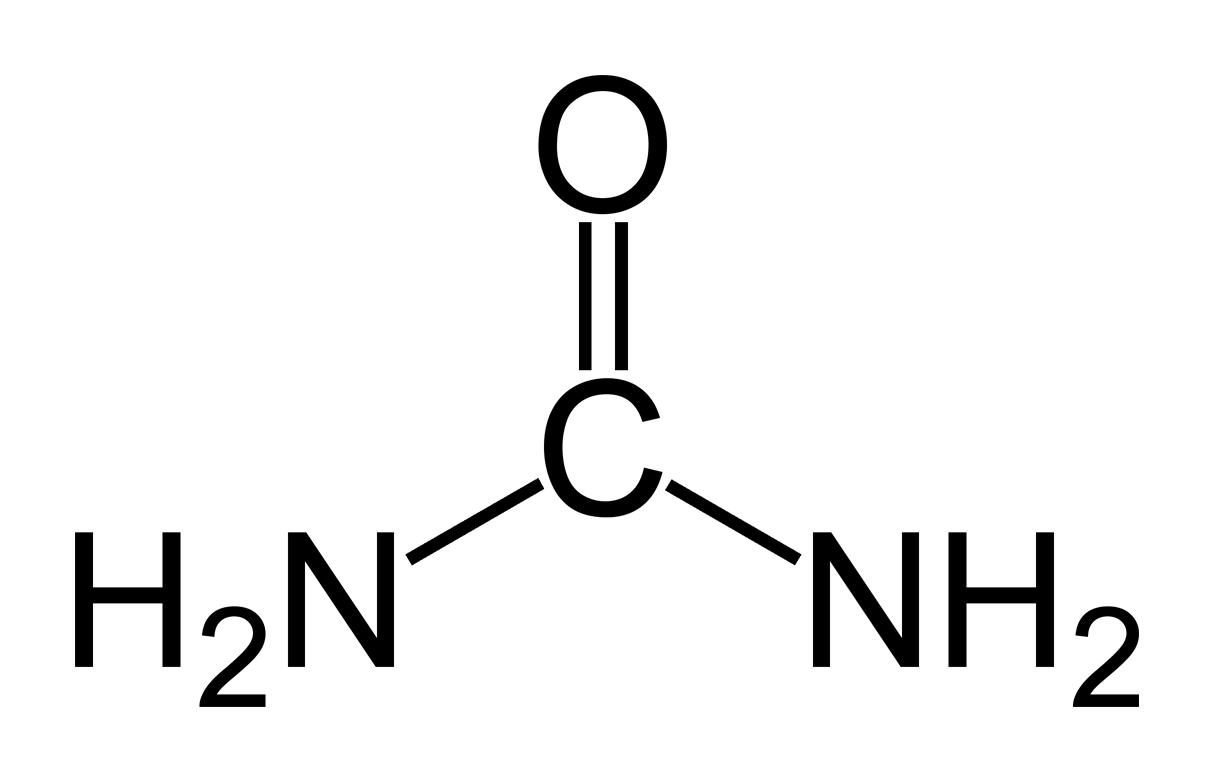
My question is about how to choose the solvent (from among mercaptoethanol, urea, or GuHCl) to create a favorable environment for activity of a hydrophobic enzyme. I think I'm not understanding this question because I don't really get how these solvents would (or would not) interact with something hydrophobic.
1. I'm trying to understand this in terms of like-dissolves-like - is this how I should be thinking about this?
2. Since beta-galactosidase is mostly hydrophobic, do we want to use urea because it is NOT hydrophobic?
3. The explanation says urea acts primarily on hydrogen bonds and mercaptoethanol acts mainly on disulfide bonds. So urea would be less likely to interact with hydrophobic beta-galactosidase because...why?
4. And why is urea a better solvent choice than mercaptoethanol?
5. Why would GuHCl "most disrupt hydrophobic interactions"?
6. I read in some abstract that turned up in my search that urea and GuHCl are non-polar. But it looks polar. So which is it?
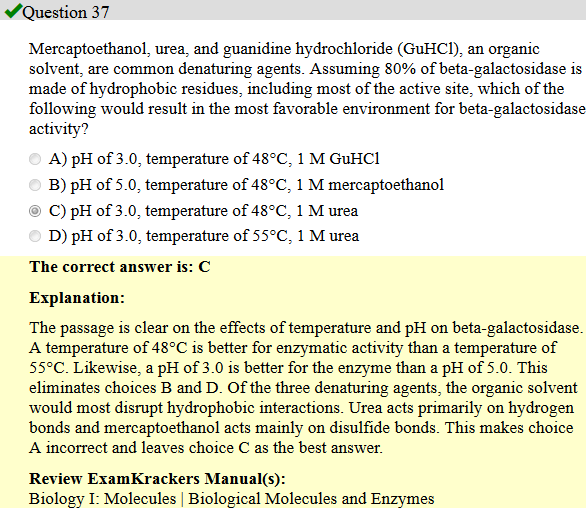
1. I'm trying to understand this in terms of like-dissolves-like - is this how I should be thinking about this?
2. Since beta-galactosidase is mostly hydrophobic, do we want to use urea because it is NOT hydrophobic?
3. The explanation says urea acts primarily on hydrogen bonds and mercaptoethanol acts mainly on disulfide bonds. So urea would be less likely to interact with hydrophobic beta-galactosidase because...why?
4. And why is urea a better solvent choice than mercaptoethanol?
5. Why would GuHCl "most disrupt hydrophobic interactions"?
6. I read in some abstract that turned up in my search that urea and GuHCl are non-polar. But it looks polar. So which is it?