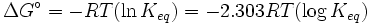- Joined
- Mar 28, 2009
- Messages
- 101
- Reaction score
- 0
OK question from kaplan
what is the equilibrium constant for a SPONTANEOUS reaction moving in the forward direction?
I thought it was misworded... cant this be k> 1 or k< 1 or k=1
seems like you need more info
what is the equilibrium constant for a SPONTANEOUS reaction moving in the forward direction?
I thought it was misworded... cant this be k> 1 or k< 1 or k=1
seems like you need more info