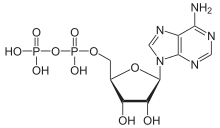
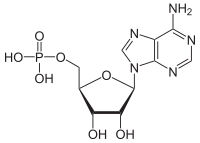
How does the free energy (G) of a system containing one mole of ADP compare to the free energy of a system containing one mole of AMP under standard physiological conditions?
hi guys I dont get why ADP has more positive free energy compared to AMP. according to Khan "The free energy of a system can never be negative. Remember, free energy (G) is not the same as free energy change (∆G)."
hi guys I dont get why ADP has more positive free energy compared to AMP. according to Khan "The free energy of a system can never be negative. Remember, free energy (G) is not the same as free energy change (∆G)."