- Joined
- Oct 29, 2010
- Messages
- 5,348
- Reaction score
- 20
Given the structure below, the hybridization, electronic geometry, and observable geometry of the oxygen atom is:
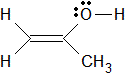
A
sp2, trigonal planar, trigonal planar
B
sp2, trigonal planar, bent
C
sp3, tetrahedral, tetrahedral
D
sp3, tetrahedral, trigonal pyramidal
I thought it would be sp3 since there are no pi bonds on the oxygen atom and Tetrahedral because the two lone pairs and two sigma bonds and thus the answer would be D. Can someone please explain why its B.

A
sp2, trigonal planar, trigonal planar
B
sp2, trigonal planar, bent
C
sp3, tetrahedral, tetrahedral
D
sp3, tetrahedral, trigonal pyramidal
I thought it would be sp3 since there are no pi bonds on the oxygen atom and Tetrahedral because the two lone pairs and two sigma bonds and thus the answer would be D. Can someone please explain why its B.
