Once again, I see eye to eye with neglect who is always realistic and clear-thinking. As far as I am concerned, I have absolutely no confidence the stroke community. I am completely cynical. They will not respond to negative data. They are just like cardiologists. The negative data will continue to roll in, and the number of procedures will continue to increase. I actually disagree with neglect about INR being a dying field because people will continue to do off-label and essentially contraindicated procedures out of desperation. It is the same psychology of not giving up on an elderly, irreversibly ill and suffering relative or giving chemotherapy to an 85 year old with multiple comorbidities. We are men of action. Action implies strength. Inaction implies ineptitude and apathy.
I don't care if strokeguy has 30 years of experience and 100 publications. These procedures are failing misreably. Your proceduralists are not better than those in these clinical trials. Your clinical judgment is not significantly better than the neurologists in these trials. Get over yourself.
In some of these trials, the deck was stacked heavily in favor of intervention. Think about IMS3 and how much better these patients were as candidates for intervention compared to the typical patient getting off -label thrombectomy (i.e 6.5 hours out, a lot of abnormalities on NC CT)
I actually would credit stroke guy for making very reasonable statements later in this thread, espcially this:
In my opinion this should at least make physicians think that unless something gets proven in well designed clinical trials it is a falacy to make it a pseudo-standard of care.
Here are some of my responses to statements made earlier in this thread
Unlike medical therapies, devices are extremely technology dependent.
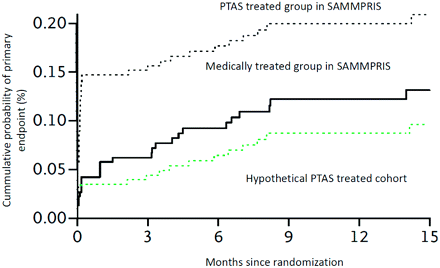
stenting went head to head against nothing and was utterly destroyed.
Future clinically trials should be in patients with strokes despite maximum medical therapy and done with extreme caution and pessimism.
Lets say, we dont want to stent, then also there is always a safer option of balloon angioplasty alone.
Prove it in a clinical trial first.
Many people are not aware of the results of SENTIS.
Interesting. Perhaps this would be especially beneficial in patients with low blood pressure and/or heart failure
Let me also clarify CREST did NOT show that surgery is better than stenting. CREST demonstrated NON-INFERIORITY of stenting compared to surgery.
Oh really?
check out the article if you questions the statistical significance:
http://www.ncbi.nlm.nih.gov/pubmed/23265585
The article says "When performed by surgeons, CAS and CEA have similar net outcomes, although the periprocedural risks vary (lower stroke with CEA and lower MI with CAS). These data suggest that appropriately trained vascular surgeons may safely offer both CEA and CAS for the prevention of stroke." However, CEA was clearly better in preventing stroke, though CAS in this study did not entirely have modern protection devices
If you all remember that prior trials (including European) showed stenting inferior to surgery. This again has to do with evolution of technology; newer generation devices were used in CREST. This should be kept in mind with regards to intracranial stenting as well.
All wishful thinking. Prove it in a clinical trial.
The more acute stroke cases that go to interventional, the more M&M. This could not have been further from the truth.
You've lost your mind. Interventional treatment causes M&M cases. I've seen so many. I've also seen some younger pts with M1 syndromes do surprising well when they weren't treated because they didn't develop reperfusion injury, massive cerebral edema, prolonged hospital stays, complications et cetera.