- Joined
- May 21, 2014
- Messages
- 30
- Reaction score
- 16
I have a few questions from the Kaplan 3R practice test and I would really appreciate any help.
51. 1M NaOH is added to a solution containing 1M Ag+,
1M Al3+, 1M Mg2+, and 1M Mn2+. Given the solubility
data shown below, which of the following will
precipitate first?
Ksp
AgOH 1.5 × 10–8
Al(OH)3 3.7 × 10–15
Mg(OH)2 1.2 × 10–11
Mn(OH)2 2.0 × 10–13
A. AgOH
B. Al(OH)3
C. Mg(OH)2
D. Mn(OH)2
Answer:
A
The question asks which will precipitate first. Each metal hydroxide, M(OH)n, has an equilibrium
equation of the following form:
M(OH)n ==> M(n+) + nOH– --------where M is a metal, n is a subscript of M
Ksp = [Mn+][OH–]^n-----------n is a subscript of M. This is not manganese
Since the concentration of each metal [M] = 1 molar, the equilibrium equation reduces to:
Ksp = [OH–]^n
Thus, the saturation concentration of hydroxide ion, in each case, is:
[OH–] = n√ Ksp
-----------------Ksp----------------------saturation [OH–]
AgOH ---------1.5 × 10–8-------------1.5 × 10–8
Al(OH)3--------3.7 × 10–15-----------1.5 × 10–5
Mg(OH)2-------1.2 × 10–11-----------3.4 × 10–6
Mn(OH)2-------2.0 × 10–13-----------4.5 × 10–7
AgOH is saturated at the lowest [OH–] and will therefore precipitate first.
Comments: Isn't the derived Ksp supposed to be [n OH]^n? Or am I missing something here?
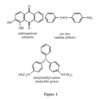
152. Cotton is a natural fiber composed of cellulose, a
polymer of glucose. Which of the compounds shown
in Figure 1 (figure 1 is labeled "hydrogen bonding" below) would adhere to a cotton fiber via hydrogen
bonding?
A. Alizarin only
B. Malachite green only
C. Alizarin and aniline yellow only
D. Malachite green, alizarin, and aniline yellow
Answer:
C
Hydrogen bonding occurs between OH groups and NH groups in any combination. Since the
glucose monomer in cellulose has OH groups, cellulose should be capable of hydrogen bonding
to either alizarin, with its two OH groups, or to aniline yellow, with its aromatic amine functionality.
Malachite green, on the other hand, has no acidic protons available for hydrogen bonding
due to the methylation of the nitrogen atoms. (The figure showing the relevant compounds for this question is labeled "Kaplan 2" below)
Comment: Nitrogens with lone pairs will not attract partially positive hydrogens in a compound like say H2O?! I looked up various sources and compounds with nitrogens not bonded to Hs CAN hydrogen bond
181.Which of the following ketones will have the most
acidic α-hydrogen:
Answer:
B
Alpha hydrogens are relatively acidic because of resonance stabilization as shown below. The
acidity of the alpha hydrogens will be increased by proximity to electron withdrawing groups. (The resonance structure being referred to here is the one where the oxygen bonded to the carbonyl takes on a (-) charge).
The chloride groups in choice B are electron withdrawing and will help stabilize the negative
charge.
Choice A is incorrect because dimethylketone has no additional electron withdrawing
groups.
Choice C is incorrect because an amine is an electron donating group and would tend to
decrease the acidity of the alpha hydrogen.
Choice D is incorrect because there is no alpha hydrogen to be removed.
The Cl is 2 carbons away while the nitrogen is right next to the hydrogen. The lone pairs resulting from deprotonation will be resonance stabilized so how does the Kaplan answer make sense?
51. 1M NaOH is added to a solution containing 1M Ag+,
1M Al3+, 1M Mg2+, and 1M Mn2+. Given the solubility
data shown below, which of the following will
precipitate first?
Ksp
AgOH 1.5 × 10–8
Al(OH)3 3.7 × 10–15
Mg(OH)2 1.2 × 10–11
Mn(OH)2 2.0 × 10–13
A. AgOH
B. Al(OH)3
C. Mg(OH)2
D. Mn(OH)2
Answer:
A
The question asks which will precipitate first. Each metal hydroxide, M(OH)n, has an equilibrium
equation of the following form:
M(OH)n ==> M(n+) + nOH– --------where M is a metal, n is a subscript of M
Ksp = [Mn+][OH–]^n-----------n is a subscript of M. This is not manganese
Since the concentration of each metal [M] = 1 molar, the equilibrium equation reduces to:
Ksp = [OH–]^n
Thus, the saturation concentration of hydroxide ion, in each case, is:
[OH–] = n√ Ksp
-----------------Ksp----------------------saturation [OH–]
AgOH ---------1.5 × 10–8-------------1.5 × 10–8
Al(OH)3--------3.7 × 10–15-----------1.5 × 10–5
Mg(OH)2-------1.2 × 10–11-----------3.4 × 10–6
Mn(OH)2-------2.0 × 10–13-----------4.5 × 10–7
AgOH is saturated at the lowest [OH–] and will therefore precipitate first.
Comments: Isn't the derived Ksp supposed to be [n OH]^n? Or am I missing something here?
152. Cotton is a natural fiber composed of cellulose, a
polymer of glucose. Which of the compounds shown
in Figure 1 (figure 1 is labeled "hydrogen bonding" below) would adhere to a cotton fiber via hydrogen
bonding?
A. Alizarin only
B. Malachite green only
C. Alizarin and aniline yellow only
D. Malachite green, alizarin, and aniline yellow
Answer:
C
Hydrogen bonding occurs between OH groups and NH groups in any combination. Since the
glucose monomer in cellulose has OH groups, cellulose should be capable of hydrogen bonding
to either alizarin, with its two OH groups, or to aniline yellow, with its aromatic amine functionality.
Malachite green, on the other hand, has no acidic protons available for hydrogen bonding
due to the methylation of the nitrogen atoms. (The figure showing the relevant compounds for this question is labeled "Kaplan 2" below)
Comment: Nitrogens with lone pairs will not attract partially positive hydrogens in a compound like say H2O?! I looked up various sources and compounds with nitrogens not bonded to Hs CAN hydrogen bond
181.Which of the following ketones will have the most
acidic α-hydrogen:
Answer:
B
Alpha hydrogens are relatively acidic because of resonance stabilization as shown below. The
acidity of the alpha hydrogens will be increased by proximity to electron withdrawing groups. (The resonance structure being referred to here is the one where the oxygen bonded to the carbonyl takes on a (-) charge).
The chloride groups in choice B are electron withdrawing and will help stabilize the negative
charge.
Choice A is incorrect because dimethylketone has no additional electron withdrawing
groups.
Choice C is incorrect because an amine is an electron donating group and would tend to
decrease the acidity of the alpha hydrogen.
Choice D is incorrect because there is no alpha hydrogen to be removed.
The Cl is 2 carbons away while the nitrogen is right next to the hydrogen. The lone pairs resulting from deprotonation will be resonance stabilized so how does the Kaplan answer make sense?
Attachments
Last edited: