- Joined
- May 17, 2008
- Messages
- 3,022
- Reaction score
- 3,617
Hey guys, I made this post over in the DAT section but I thought I would put it here as well since the same concepts are found on the MCAT:
This isn't actually a question of mine, but an answer to a question I have seen many times and often had trouble with. Here is a (lengthy) writeup with images that I believe will clear everything up:
A noncompetitive inhibitor is defined as: "a substance that inhibits the action of an enzyme by binding to the enzyme at a location other than the active site."
Allosteric inhibition is defined as: "a substance that binds to the enzyme and induces the enzyme's inactive form."
These definitions appear extremely similar, so what is the difference, and why do we distinguish between these two concepts? The following is an illustrated example of noncompetitive inhibition:
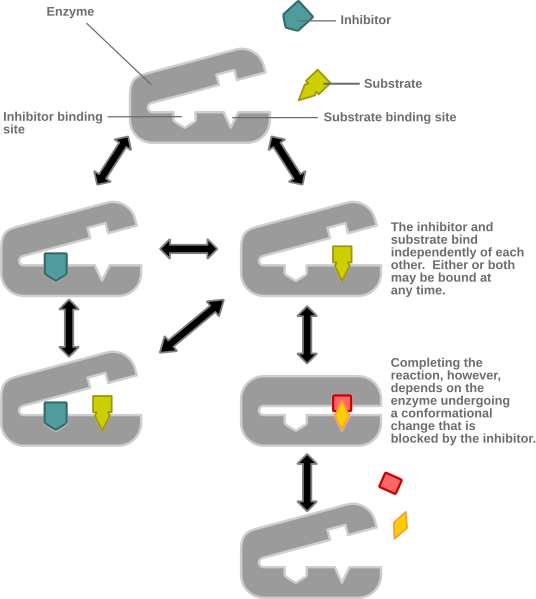
Note that it does NOT prevent the substrate from binding to the active site, but it still prevents the reaction from completing. Also note that the noncompetitive inhibitor binds at an allosteric site. This is key to understanding the difference: all noncompetitive inhibition is allosteric inhibition, but not all allosteric inhibition is noncompetitive inhibition. Why? Because certain forms of allosteric inhibition can prevent the substrate from binding to the active site, in others words, allosteric inhibition can be noncompetitive or competitive. The above picture is an example of allosteric noncompetitive inhibition. Below is an example of allosteric competitive inhibition:

Another example of allosteric competitive inhibition below:
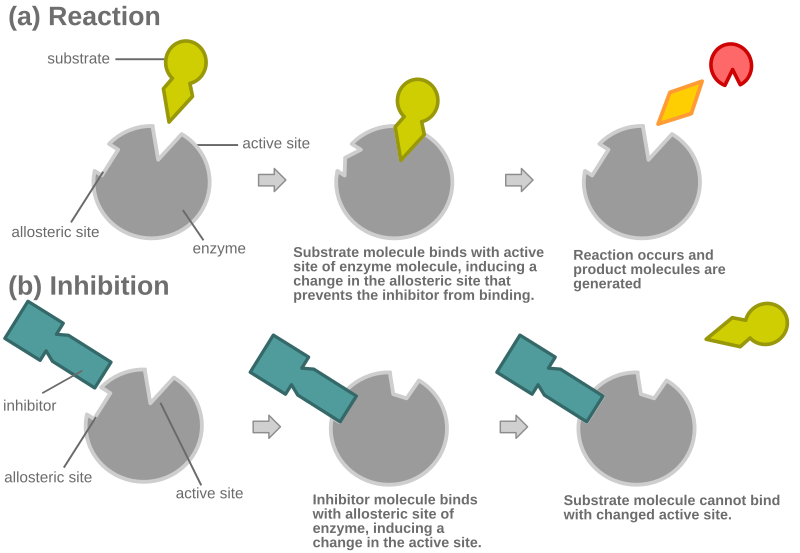
For contrast, here is standard (non-allosteric) competitive inhibition:

With this in mind, we can now understand why allosteric inhibition is a broad concept that does not follow specific Vmax or Km trends like standard competitive and noncompetitive inhibition do, because it can refer to a variety of conditions under which the substrate may or may not be able to bind to the active site.
It is also worth noting that if you use Barron's AP Biology review book, as of the 3rd edition, it has a completely incorrect definition of noncompetitive inhibitors.
Please let me know if this helped you out, or if I can make it any clearer.
This isn't actually a question of mine, but an answer to a question I have seen many times and often had trouble with. Here is a (lengthy) writeup with images that I believe will clear everything up:
A noncompetitive inhibitor is defined as: "a substance that inhibits the action of an enzyme by binding to the enzyme at a location other than the active site."
Allosteric inhibition is defined as: "a substance that binds to the enzyme and induces the enzyme's inactive form."
These definitions appear extremely similar, so what is the difference, and why do we distinguish between these two concepts? The following is an illustrated example of noncompetitive inhibition:

Note that it does NOT prevent the substrate from binding to the active site, but it still prevents the reaction from completing. Also note that the noncompetitive inhibitor binds at an allosteric site. This is key to understanding the difference: all noncompetitive inhibition is allosteric inhibition, but not all allosteric inhibition is noncompetitive inhibition. Why? Because certain forms of allosteric inhibition can prevent the substrate from binding to the active site, in others words, allosteric inhibition can be noncompetitive or competitive. The above picture is an example of allosteric noncompetitive inhibition. Below is an example of allosteric competitive inhibition:

Another example of allosteric competitive inhibition below:

For contrast, here is standard (non-allosteric) competitive inhibition:

With this in mind, we can now understand why allosteric inhibition is a broad concept that does not follow specific Vmax or Km trends like standard competitive and noncompetitive inhibition do, because it can refer to a variety of conditions under which the substrate may or may not be able to bind to the active site.
It is also worth noting that if you use Barron's AP Biology review book, as of the 3rd edition, it has a completely incorrect definition of noncompetitive inhibitors.
Please let me know if this helped you out, or if I can make it any clearer.
