Can someone explain this (maybe with a drawing)?
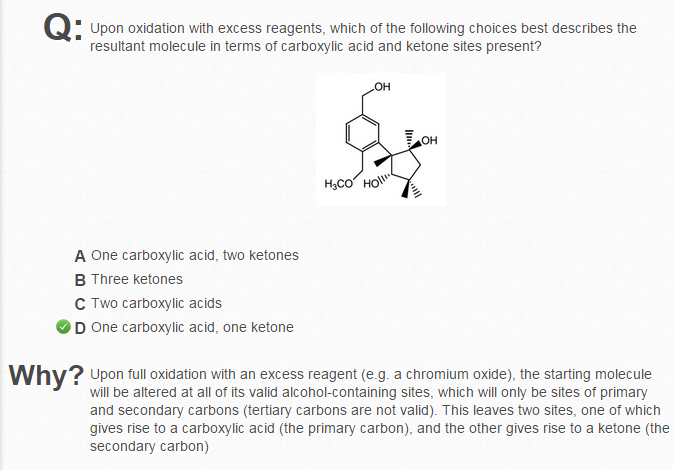
I understand that during oxidation, all the OH groups will become C=O, I thought there would be 3 ketones.
I understand that during oxidation, all the OH groups will become C=O, I thought there would be 3 ketones.
