Orgo is hard...
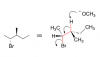
This attached pic. I tried drawing it, and picturing it in my head and cannot for the life of me admit that the two are the same..Lets call the carbon on the left side carbon #1. It has bromine attached to it which is going into the plane, and a methyl group on the plane. I visualized the rotation of the bromine group into the plane, and with that rotation, the methyl group rotates into the plane and the hydrogen is on the plane. All good so far. The problem is that when the whole molecule is rotated in that specific way, the other carbon #2 seems completely wrong. The methyl that was originally sticking out of the plane should now be on the plane (but it is still out of the plane), and the ethyl group should be out of the plane but it is into the plane, etc.
Does anyone get what I'm saying here? I'm not sure if I'm just absolutely wrong..
This attached pic. I tried drawing it, and picturing it in my head and cannot for the life of me admit that the two are the same..Lets call the carbon on the left side carbon #1. It has bromine attached to it which is going into the plane, and a methyl group on the plane. I visualized the rotation of the bromine group into the plane, and with that rotation, the methyl group rotates into the plane and the hydrogen is on the plane. All good so far. The problem is that when the whole molecule is rotated in that specific way, the other carbon #2 seems completely wrong. The methyl that was originally sticking out of the plane should now be on the plane (but it is still out of the plane), and the ethyl group should be out of the plane but it is into the plane, etc.
Does anyone get what I'm saying here? I'm not sure if I'm just absolutely wrong..