- Joined
- May 27, 2011
- Messages
- 3,486
- Reaction score
- 82
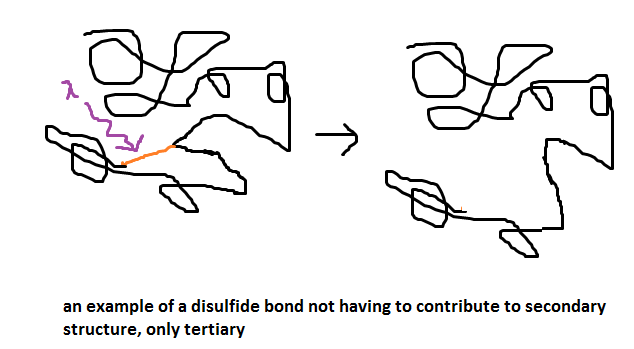
Taking a protein from a lipid environment and placing it into an aqueous environment would most likely affect which of the following?
A. Primary structure
B. Secondary structure
C. Tertiary structure
D. Quaternary structure
Can someone help me visualize that stuff?
A. Primary structure
B. Secondary structure
C. Tertiary structure
D. Quaternary structure
Can someone help me visualize that stuff?