- Joined
- May 19, 2010
- Messages
- 17
- Reaction score
- 2
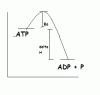
When ATP is hydrolyzed to ADP + Pi, is this an endothermic/ exothermic reaction? Also, is it exergonic/ energonic? I saw a destroyer problem that stated that hydrolysis of ATP is exergonic. But breaking a bond requires energy, so would that mean ATP hydrolysis is endothermic AND exergonic??? Please help, thanks!