- Joined
- Jun 17, 2013
- Messages
- 152
- Reaction score
- 66
Hey folks, I've included an image of this problem below. I can't seem to understand the correct answer.
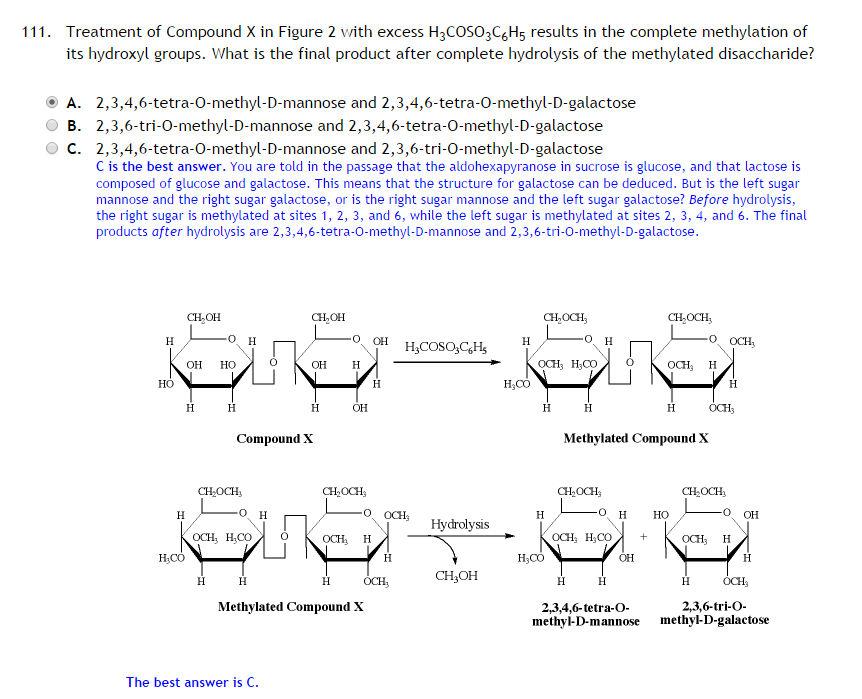
After hydrolysis, according to this, the anomeric carbon of galactose is turned from a methoxy group to a hydroxyl group, thereby reducing the number of methylations to two. The same does not happen to C4 of mannose, or any of the other hydroxyl groups. Is it solely because the carbon is anomeric? I'm having a little trouble understanding their reasoning here.
Cheers!
Edit:
I have one more question that should be simple, but I'm somewhat stumped on regarding stereochemistry.
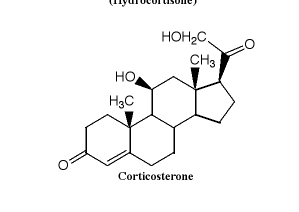
According to the answer to a question, both quaternary carbons have have S-stereochemistry. I feel like I'm having trouble grouping the substituents when they're ringed, and when there are so many carbons. How would you go about ordering them?
Thanks again!

After hydrolysis, according to this, the anomeric carbon of galactose is turned from a methoxy group to a hydroxyl group, thereby reducing the number of methylations to two. The same does not happen to C4 of mannose, or any of the other hydroxyl groups. Is it solely because the carbon is anomeric? I'm having a little trouble understanding their reasoning here.
Cheers!
Edit:
I have one more question that should be simple, but I'm somewhat stumped on regarding stereochemistry.

According to the answer to a question, both quaternary carbons have have S-stereochemistry. I feel like I'm having trouble grouping the substituents when they're ringed, and when there are so many carbons. How would you go about ordering them?
Thanks again!
Last edited:
