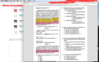
Would someone provide a better explanation for solving this problem, and the "mathematical" and conceptual understanding that aids in solving this problem? I "kinda" get it, but I don't "strongly" get it. Question is attached in screenshot, and pasted below:
47.. Which of the following kinetic relationships is predicted for Reaction 1 using steady-state approximation?
A. ki[[(NH3)5CoCl]2+][OH-]
= (k., + k2) [[(NH3)4Co(NH2)Cl]+] [H20]
B. kj[[(NH3)5CoCl]2+][OH-]
= (k., + k2) [[(NH3)4Co(NH2)Cl]+]
C. kj[[(NH3)5CoCl]2+][OH-]= k., [[(NH3)4Co(NH2)Cl]+] [H20]
+ k2[[(NH3)4Co(NH2)Cl]+]
D. ki [[(NH3)5CoCl]2+][OH-]
= k.,[[(NH3)4Co(NH2)Cl]+]
+ k2 [[(NH3)4Co(NH2)Cl]+] [H20]
EXPLANATION:
47.. Which of the following kinetic relationships is predicted for Reaction 1 using steady-state approximation?
A. ki[[(NH3)5CoCl]2+][OH-]
= (k., + k2) [[(NH3)4Co(NH2)Cl]+] [H20]
B. kj[[(NH3)5CoCl]2+][OH-]
= (k., + k2) [[(NH3)4Co(NH2)Cl]+]
C. kj[[(NH3)5CoCl]2+][OH-]= k., [[(NH3)4Co(NH2)Cl]+] [H20]
+ k2[[(NH3)4Co(NH2)Cl]+]
D. ki [[(NH3)5CoCl]2+][OH-]
= k.,[[(NH3)4Co(NH2)Cl]+]
+ k2 [[(NH3)4Co(NH2)Cl]+] [H20]
EXPLANATION:
- The steady state approximation says that after an initial period of time, balance within the reaction takes place, and the concentration of any intermediates stay relatively constant (in a steady state). This means that the rate at which the intermediate is produced is equal to the rate at which it is consumed. The intermediate in this case is [(NH3)4Co(NH2)Cl]+, which is held constant by being in the rate- determining step. It is formed by the forward reaction in Step I and consumed by both the reverse reaction of Step I and the forward reaction of Step II. Water is involved only in the reverse reaction of Step I, so water must appear only with k_i- This eliminates all choices except C, which is the correct choice. In choice C, the
rate law for the reaction that forms the intermediate is set equal to the sum of the rate law for the reverse reaction of Step I and the forward reaction of Step II.