Sorry for my many questions. I got the following questions correct by logically thinking it out, but I thought I should understand how to calculate the answers?
30. By roughly how much do the two equivalents points in the first graph differ?
A. Fewer than 2.0 pH units
B. Fewer than 4.0 pH units, but more than 2.0 pH units
C. Fewer than 8.0 pH units, but more than 4.0 pH units
D. More than 8.0 pH units
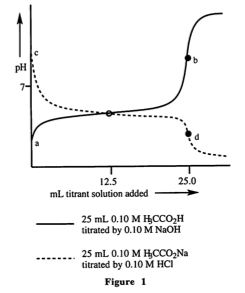
I looked at the graph and I roughly estimated the numbers and got the correct answer, but I don't understand how they went about calculating the problem.

How do they figure out that it should be 0.05 M HoAc and 0.05 M OAc-. And then how do they assume that pKa is roughly 5.0?
31. The pH at equivalence is GREATEST for which of the following titrations?
A. The titration of 0.10 M H3CCO2H by NaOH
B. The titration of 0.10 M H3CCO2Na by HCl
C. The titration o f 0.10 M CH3NH3CI by NaOH
D. The titration of 0.10 M CH3NH2 by HCl

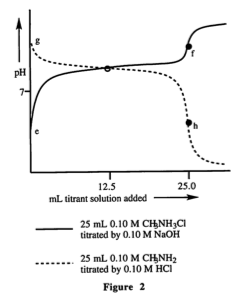
I eliminated B and D because the pH wouldn't be highest with HCl being titrated. Then I just looked at the graphs and the equivalence point for CH3NH3Cl looked higher so I picked that, but i wanted to understand their reasoning to be safe.

I don't understand why they start talking about the conjugate base and all that after the "(pH at equivalence is less than 7.0)."
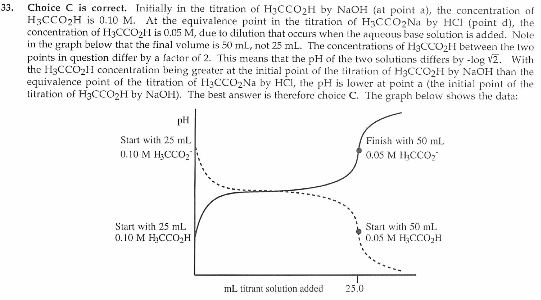
33. How does the pH at point a in Figure 1 compare to the pH at point d in Figure 1?
A . The pH at point a is more than 1.0 pH unit greater than the pH at point d.
B. The pH at point a is greater than the pH at point d, but the difference is less than one pH unit.
C. The pH at point a is less than the pH at point d, but the difference is less than one pH unit.
D. The pH at point a is more than 1.0 pH unit lower than the pH at point d.

I looked at the graph and a and d seemed very close, so it had to be within 1 pH unit.
30. By roughly how much do the two equivalents points in the first graph differ?
A. Fewer than 2.0 pH units
B. Fewer than 4.0 pH units, but more than 2.0 pH units
C. Fewer than 8.0 pH units, but more than 4.0 pH units
D. More than 8.0 pH units
I looked at the graph and I roughly estimated the numbers and got the correct answer, but I don't understand how they went about calculating the problem.
How do they figure out that it should be 0.05 M HoAc and 0.05 M OAc-. And then how do they assume that pKa is roughly 5.0?
31. The pH at equivalence is GREATEST for which of the following titrations?
A. The titration of 0.10 M H3CCO2H by NaOH
B. The titration of 0.10 M H3CCO2Na by HCl
C. The titration o f 0.10 M CH3NH3CI by NaOH
D. The titration of 0.10 M CH3NH2 by HCl
I eliminated B and D because the pH wouldn't be highest with HCl being titrated. Then I just looked at the graphs and the equivalence point for CH3NH3Cl looked higher so I picked that, but i wanted to understand their reasoning to be safe.
I don't understand why they start talking about the conjugate base and all that after the "(pH at equivalence is less than 7.0)."
33. How does the pH at point a in Figure 1 compare to the pH at point d in Figure 1?
A . The pH at point a is more than 1.0 pH unit greater than the pH at point d.
B. The pH at point a is greater than the pH at point d, but the difference is less than one pH unit.
C. The pH at point a is less than the pH at point d, but the difference is less than one pH unit.
D. The pH at point a is more than 1.0 pH unit lower than the pH at point d.
I looked at the graph and a and d seemed very close, so it had to be within 1 pH unit.
