20. Why is the initial rate observed in reaction studies?
A. As a reaction proceeds, it slows. The same period in the reaction must be compared to be consistent.
B. As a reaction proceeds, its rate increases. The same period in the reaction must be compared to be consistent.
C. Only the initial rate can be accurately measured.
D. Any rate can be measured, but the initial one is most convenient.
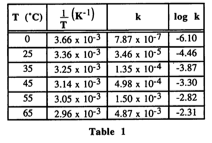

Looking at the table, k (rate constant) is increasing as the temperature increases, so I don't understand why the rate would be decreasing? I picked, B, but the answer is A. Am I supposed to be looking at 1/T or something?
A. As a reaction proceeds, it slows. The same period in the reaction must be compared to be consistent.
B. As a reaction proceeds, its rate increases. The same period in the reaction must be compared to be consistent.
C. Only the initial rate can be accurately measured.
D. Any rate can be measured, but the initial one is most convenient.
Looking at the table, k (rate constant) is increasing as the temperature increases, so I don't understand why the rate would be decreasing? I picked, B, but the answer is A. Am I supposed to be looking at 1/T or something?
