- Joined
- May 14, 2014
- Messages
- 806
- Reaction score
- 641
The question reads:
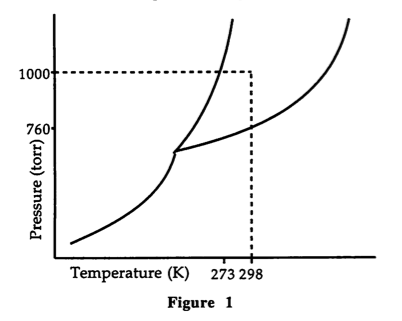
Which of the following conclusions is INVALID for Compound A?
A. Compound A does not sublime at 25°C.
B. Compound A is solid at 250 K, 760 torr.
C. Compound A has a triple point roughly equal to that of water.
D. For Compound A there exists a solid that is less dense than its liquid phase.
Choice D is correct. Compound A does sublime at 25°C (298 K), if at a pressure below 760 torr; Compound A is a solid at 250 K and 760 torr; and Compound A does have a triple point roughly equal to water (the triple point of water is at 0°C). The only uncertainty is the relative densities of the solid and liquid phases. There is no point at which an increase in pressure at constant temperature converts the solid to a liquid. An increase in the pressure increases the density of the material. This makes choice D the best choice.
Why isn't A an invalid statement?

Which of the following conclusions is INVALID for Compound A?
A. Compound A does not sublime at 25°C.
B. Compound A is solid at 250 K, 760 torr.
C. Compound A has a triple point roughly equal to that of water.
D. For Compound A there exists a solid that is less dense than its liquid phase.
Choice D is correct. Compound A does sublime at 25°C (298 K), if at a pressure below 760 torr; Compound A is a solid at 250 K and 760 torr; and Compound A does have a triple point roughly equal to water (the triple point of water is at 0°C). The only uncertainty is the relative densities of the solid and liquid phases. There is no point at which an increase in pressure at constant temperature converts the solid to a liquid. An increase in the pressure increases the density of the material. This makes choice D the best choice.
Why isn't A an invalid statement?
