- Joined
- Dec 14, 2011
- Messages
- 6
- Reaction score
- 0
Hi all -
I had a question regarding TBR's Electrolysis passage from their old books:
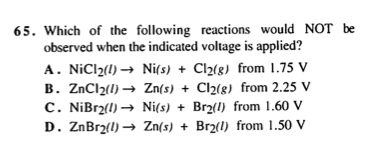
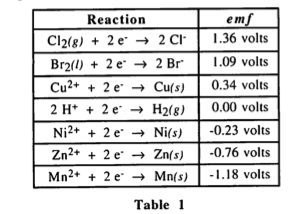
My question is this: The table suggests that Cl2(g) has a stronger reduction potential than Ni(2+) so it makes sense that Cl(2) would be the one that is becoming reduced and Ni(2+) will have to be reversed (oxidized) - thereby making its emf + 0.23 and adding it with 1.36. That's basically what the solution to this Q suggests.
However - from the question that was given - Cl2(g) is shown to be the product. Does that not mean that we have to reverse the Cl2(g) + 2e- rxn instead and keep Ni2+ the way that it is?
Many thanks for your help!
I had a question regarding TBR's Electrolysis passage from their old books:
My question is this: The table suggests that Cl2(g) has a stronger reduction potential than Ni(2+) so it makes sense that Cl(2) would be the one that is becoming reduced and Ni(2+) will have to be reversed (oxidized) - thereby making its emf + 0.23 and adding it with 1.36. That's basically what the solution to this Q suggests.
However - from the question that was given - Cl2(g) is shown to be the product. Does that not mean that we have to reverse the Cl2(g) + 2e- rxn instead and keep Ni2+ the way that it is?
Many thanks for your help!
