- Joined
- May 14, 2014
- Messages
- 806
- Reaction score
- 641
The question asks:
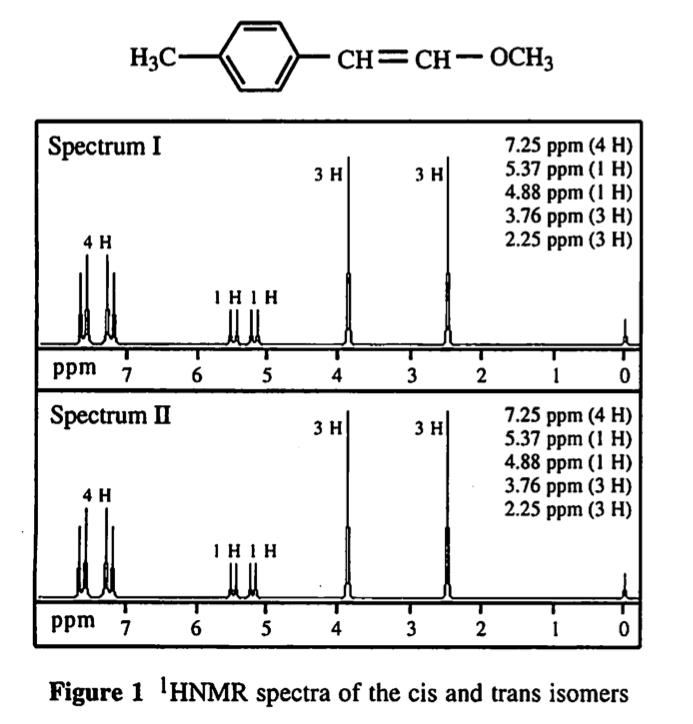
58. Spectrum 1 is associated with the:
A. trans compound, because the J value is smaller
B. cis compound, because the J value is smaller
C. trans compound, because the J value is larger
D. cis compound, because the J value is larger
Choice C is correct. In Spectrum I, the distance between the peaks in the alkene region (coupling constant) is greater than it is in Spectrum II. A larger coupling between vinylic hydrogens is attributed to the trans compound. This is choice C.
I am just not seeing it. Can someone please help explain this to me? I don't see a difference in any distances in the figure.

58. Spectrum 1 is associated with the:
A. trans compound, because the J value is smaller
B. cis compound, because the J value is smaller
C. trans compound, because the J value is larger
D. cis compound, because the J value is larger
Choice C is correct. In Spectrum I, the distance between the peaks in the alkene region (coupling constant) is greater than it is in Spectrum II. A larger coupling between vinylic hydrogens is attributed to the trans compound. This is choice C.
I am just not seeing it. Can someone please help explain this to me? I don't see a difference in any distances in the figure.
