- Joined
- Feb 23, 2009
- Messages
- 640
- Reaction score
- 104
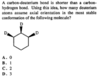
Can someone explain to me how the answer is C? I thought the more equatorial deuterium the better, but the explanation says "The most stable orientation has as many deuterium atoms with axial orientation possible."
Lost.
Thanks!
Lost.
Thanks!