- Joined
- Jul 6, 2006
- Messages
- 42
- Reaction score
- 0
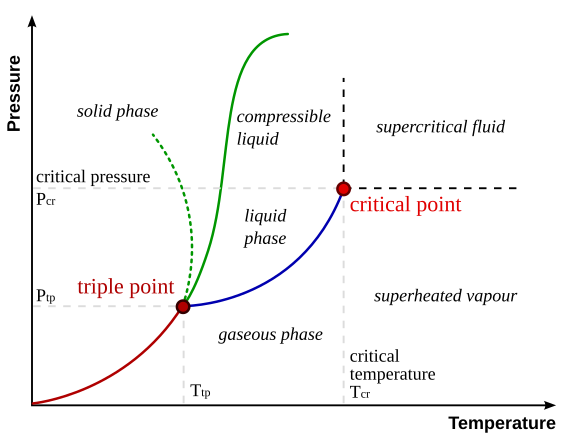
I'm confused over when a substance can be in equillibrium with all three "common" phases (liquid/gas/solid). Most books say that this occurs at the triple point, but can't it occur at any point? For example, the melting point is when the vapour pressure of the liquid phase and pure solid are equal, suggesting that all three phases are in equillibrium, albeit at an equillibrium where the gas phase is very little. Any help?
I think whats messing me up is the idea of melting point. Its said that as we approach melting point, the liquid vapour pressure rises to match and then exceed the solid vapour pressure. Can anyone help me interpret this?
I think whats messing me up is the idea of melting point. Its said that as we approach melting point, the liquid vapour pressure rises to match and then exceed the solid vapour pressure. Can anyone help me interpret this?