Drug Approved. Is Disease Real?
By
ALEX BERENSON
Published: January 14, 2008
Fibromyalgia is a real disease. Or so says
Pfizer in a new television advertising campaign for Lyrica, the first medicine approved to treat the pain condition, whose very existence is questioned by some doctors.
Jamie Rector for The New York Times
Lynne Matallana, who says she has fibromyalgia, said the drugs would aid acceptance.
Related
Times Health Guide: Fibromyalgia
Prozac brought
depression into the mainstream.
But other doctors — including the one who wrote the 1990 paper that defined fibromyalgia but who has since changed his mind — say that the disease does not exist and that Lyrica and the other drugs will be taken by millions of people who do not need them.
As diagnosed, fibromyalgia primarily affects middle-aged women and is characterized by chronic, widespread pain of unknown origin. Many of its sufferers are afflicted by other similarly nebulous conditions, like
irritable bowel syndrome.
Because fibromyalgia patients typically do not respond to conventional painkillers like aspirin, drug makers are focusing on medicines like Lyrica that affect the brain and the perception of pain.
Advocacy groups and doctors who treat fibromyalgia estimate that 2 to 4 percent of adult Americans, as many as 10 million people, suffer from the disorder.
Those figures are sharply disputed by those doctors who do not consider fibromyalgia a medically recognizable illness and who say that diagnosing the condition actually worsens suffering by causing patients to obsess over aches that other people simply tolerate. Further, they warn that Lyrica’s side effects, which include severe weight gain,
dizziness and
edema, are very real, even if fibromyalgia is not.
Despite the controversy, the American College of Rheumatology, the
Food and Drug Administration and insurers recognize fibromyalgia as a diagnosable disease. And drug companies are aggressively pursuing fibromyalgia treatments, seeing the potential for a major new market.
Hoping to follow Pfizer’s lead, two other big drug companies,
Eli Lilly and
Forest Laboratories, have asked the F.D.A. to let them market drugs for fibromyalgia. Approval for both is likely later this year, analysts say.
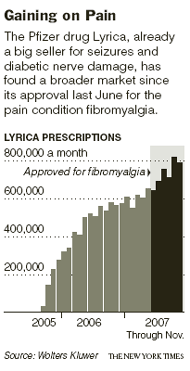
Worldwide sales of Lyrica, which is also used to treat diabetic
nerve pain and
seizures and which received F.D.A. approval in June for fibromyalgia, reached $1.8 billion in 2007, up 50 percent from 2006. Analysts predict sales will rise an additional 30 percent this year, helped by consumer advertising.
In November, Pfizer began a television ad campaign for Lyrica that features a middle-aged woman who appears to be reading from her diary. “Today I struggled with my fibromyalgia; I had pain all over,” she says, before turning to the camera and adding, “Fibromyalgia is a real, widespread pain condition.”
Doctors who specialize in treating fibromyalgia say that the disorder is undertreated and that its sufferers have been stigmatized as chronic complainers. The new drugs will encourage doctors to treat fibromyalgia patients, said Dr. Dan Clauw, a professor of medicine at the
University of Michigan who has consulted with Pfizer, Lilly and Forest.
“What’s going to happen with fibromyalgia is going to be the exact thing that happened to depression with Prozac,” Dr. Clauw said. “These are legitimate problems that need treatments.”
Dr. Clauw said that brain scans of people who have fibromyalgia reveal differences in the way they process pain, although the doctors acknowledge that they cannot determine who will report having fibromyalgia by looking at a scan.
Lynne Matallana, president of the National Fibromyalgia Association, a patients’ advocacy group that receives some of its financing from drug companies, said the new drugs would help people accept the existence of fibromyalgia. “The day that the F.D.A. approved a drug and we had a public service announcement, my pain became real to people,” Ms. Matallana said.
Ms. Matallana said she had suffered from fibromyalgia since 1993. At one point, the pain kept her bedridden for two years, she said. Today she still has pain, but a mix of drug and nondrug treatments — as well as support from her family and her desire to run the National Fibromyalgia Association — has enabled her to improve her health, she said. She declined to say whether she takes Lyrica.
“I just got to a point where I felt, I have pain but I’m going to have to figure out how to live with it,” she said. “I absolutely still have fibromyalgia.”
But doctors who are skeptical of fibromyalgia say vague complaints of chronic pain do not add up to a disease. No biological tests exist to diagnose fibromyalgia, and the condition cannot be linked to any environmental or biological causes.
The diagnosis of fibromyalgia itself worsens the condition by encouraging people to think of themselves as sick and catalog their pain, said Dr. Nortin Hadler, a rheumatologist and professor of medicine at the
University of North Carolina who has written extensively about fibromyalgia.
“These people live under a cloud,” he said. “And the more they seem to be around the medical establishment, the sicker they get.”
Dr. Frederick Wolfe, the director of the National Databank for Rheumatic Diseases and the lead author of the 1990 paper that first defined the diagnostic guidelines for fibromyalgia, says he has become cynical and discouraged about the diagnosis. He now considers the condition a physical response to
stress, depression, and economic and social anxiety.
“Some of us in those days thought that we had actually identified a disease, which this clearly is not,” Dr. Wolfe said. “To make people ill, to give them an illness, was the wrong thing.”
In general, fibromyalgia patients complain not just of chronic pain but of many other symptoms, Dr. Wolfe said. A survey of 2,500 fibromyalgia patients published in 2007 by the National Fibromyalgia Association indicated that 63 percent reported suffering from back pain, 40 percent from
chronic fatigue syndrome, and 30 percent from
ringing in the ears, among other conditions. Many also reported that fibromyalgia interfered with their daily lives, with activities like walking or climbing stairs.
Most people “manage to get through life with some vicissitudes, but we adapt,” said Dr. George Ehrlich, a rheumatologist and an adjunct professor at the
University of Pennsylvania.
“People with fibromyalgia do not adapt.”
Both sides agree that people who are identified as having fibromyalgia do not get much relief from traditional pain medicines, whether anti-inflammatory drugs like ibuprofen — sold as Advil, among other brands — or prescription opiates like Vicodin. So drug companies have sought other ways to reduce pain.
Pfizer’s Lyrica, known generically as pregabalin, binds to receptors in the brain and spinal cord and seems to reduce activity in the central nervous system.
Exactly why and how Lyrica reduces pain is unclear. In clinical trials, patients taking the drug reported that their pain — whether from fibromyalgia,
shingles or
diabetic nerve damage — fell on average about 2 points on a 10-point scale, compared with 1 point for patients taking a placebo. About 30 percent of patients said their pain fell by at least half, compared with 15 percent taking placebos.
The F.D.A. reviewers who initially examined Pfizer’s application for Lyrica in 2004 for diabetic nerve pain found those results unimpressive, especially in comparison to Lyrica’s side effects. The reviewers recommended against approving the drug, citing its side effects.
In many patients, Lyrica causes weight gain and edema, or swelling, as well as dizziness and sleepiness. In 12-week trials, 9 percent of patients saw their weight rise more than 7 percent, and the weight gain appeared to continue over time. The potential for weight gain is a special concern because many fibromyalgia patients are already overweight: the average fibromyalgia patient in the 2007 survey reported weighing 180 pounds and standing 5 feet 4 inches.
But senior F.D.A. officials overruled the initial reviewers, noting that severe pain can be incapacitating. “While pregabalin does present a number of concerns related to its potential for toxicity, the overall risk-to-benefit ratio supports the approval of this product,” Dr. Bob Rappaport, the director of the F.D.A. division reviewing the drug, wrote in June 2004.
Pfizer began selling Lyrica in the United States in 2005. The next year the company asked for F.D.A. approval to market the drug as a fibromyalgia treatment. The F.D.A. granted that request in June 2007.
Pfizer has steadily ramped up consumer advertising of Lyrica. During the first nine months of 2007, it spent $46 million on ads, compared with $33 million in 2006, according to TNS Media Intelligence.
Dr. Steve Romano, a psychiatrist and a Pfizer vice president who oversees Lyrica, says the company expects that Lyrica will be prescribed for fibromyalgia both by specialists like neurologists and by primary care doctors. As doctors see that the drug helps control pain, they will be more willing to use it, he said.
“When you help physicians to recognize the condition and you give them treatments that are well tolerated, you overcome their reluctance,” he said.
Both the Lilly and Forest drugs being proposed for fibromyalgia were originally developed as
antidepressants, and both work by increasing levels of serotonin and
norepinephrine, brain transmitters that affect mood. The Lilly drug,
Cymbalta, is already available in the United States, while the Forest drug, milnacipran, is sold in many countries, though not the United States.
Dr. Amy Chappell, a medical fellow at Lilly, said that even though Cymbalta is an antidepressant, its effects on fibromyalgia pain are independent of its antidepressant effects. In clinical trials, she said, even fibromyalgia patients who are not depressed report relief from their pain on Cymbalta.
The overall efficacy of Cymbalta and milnacipran is similar to that of Lyrica. Analysts and the companies expect that the drugs will probably be used together.
“There’s definitely room for several drugs,” Dr. Chappell said.
But physicians who are opposed to the fibromyalgia diagnosis say the new drugs will probably do little for patients. Over time, fibromyalgia patients tend to cycle among many different painkillers, sleep medicines and antidepressants, using each for a while until its benefit fades, Dr. Wolfe said.
“The fundamental problem is that the improvement that you see, which is not really great in clinical trials, is not maintained,” Dr. Wolfe said.
Still, Dr. Wolfe expects the drugs will be widely used. The companies, he said, are “going to make a fortune.”