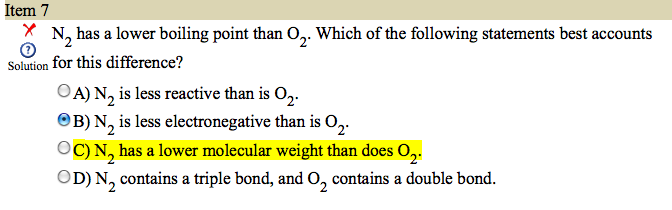EDIT: Yikes! Cant change the title. I meant boiling point not melting point
Hey guys. I got this question wrong on AAMC #4. Check out the screenshot.
It is part of a passage but IMO doesnt really have anything to do with it.
Edit: deleted pic. Sorry didn't know that was not allowed
Why is that the correct answer?
Logically I would imagine that the reason is either because O2 is more electronegative and thus can H bond more easily or because N2 has 3 bonds making it harder to break apart.
Hey guys. I got this question wrong on AAMC #4. Check out the screenshot.
It is part of a passage but IMO doesnt really have anything to do with it.
Edit: deleted pic. Sorry didn't know that was not allowed
Why is that the correct answer?
Logically I would imagine that the reason is either because O2 is more electronegative and thus can H bond more easily or because N2 has 3 bonds making it harder to break apart.
Last edited: