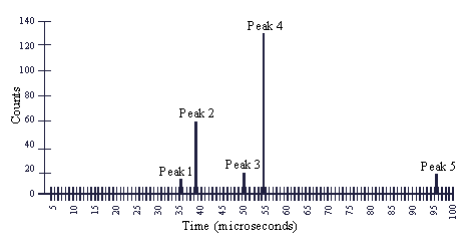
Lithium ions are shot in an Efield and the time it takes to traverse the field is measured. Peak 3 = 6-Li cation, peak 4 = 7-Li cation. So the first ones must be the corresponding double cations.
Question:
Peak 5 in figure 2 originates from a different atomic species. Given where the peak appears, and assuming that it corresponds to a singly ionized atom, we can say that the atoms of this species probably have:
A. More protons and more neutrons than 7-Li
What is the proper way to reason this question?

Question:
Peak 5 in figure 2 originates from a different atomic species. Given where the peak appears, and assuming that it corresponds to a singly ionized atom, we can say that the atoms of this species probably have:
A. More protons and more neutrons than 7-Li
What is the proper way to reason this question?
