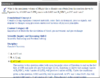
D
deleted647690
This is a basic concept that I'm getting confused about
When I first solved this, I found that .003 mol PH3 would be produced:
.002 mol R2NBCl2 * 3 mol PH3 / 2 mol R2NBCl2 tells you that .003 mol pH3 will be produced.
I thought I would need to plug this into the ideal gas law to find the final volume, thus:
V = nRT/P
.003 mol (.08) (273 K) / 1 atm ==.065 L ==65 L
However, to actually solve this, you need to know that 1 mol = 22.4 L at STP. Thus
.003 mol PH3 * 22.4 L/mol = About 67 mL, choice C
What am I confusing here? I think I'm confused about what types of questions you would use the ideal gas law for. Perhaps this was just supposed to be a stoichiometry question
When I first solved this, I found that .003 mol PH3 would be produced:
.002 mol R2NBCl2 * 3 mol PH3 / 2 mol R2NBCl2 tells you that .003 mol pH3 will be produced.
I thought I would need to plug this into the ideal gas law to find the final volume, thus:
V = nRT/P
.003 mol (.08) (273 K) / 1 atm ==.065 L ==65 L
However, to actually solve this, you need to know that 1 mol = 22.4 L at STP. Thus
.003 mol PH3 * 22.4 L/mol = About 67 mL, choice C
What am I confusing here? I think I'm confused about what types of questions you would use the ideal gas law for. Perhaps this was just supposed to be a stoichiometry question