- Joined
- Sep 19, 2013
- Messages
- 72
- Reaction score
- 15
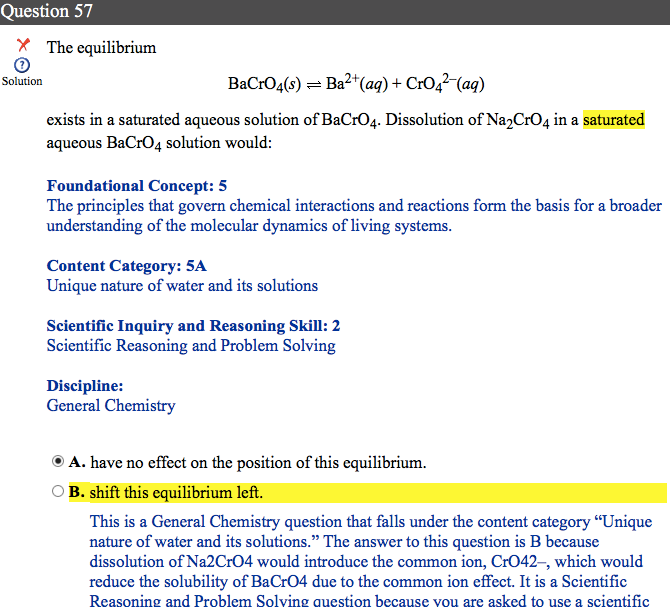
I have seen others post about this question, and I completely understand why B is the answer - same Le Chatelier principle. My issue with this question is the wording, and I'd like to ask whether this is the way that I can expect see equilibria referenced in Le Chatelier-related questions on the real MCAT.
As I understand it, the addition of new solute that with an equilibria-involved component (CrO4 2-) alters the Q value/relative concentrations transiently, before this is counteracted per Le Chatelier.
I do not agree that "the equilibria shifted," as this may only occur upon changes in temperature. Maybe I'm being pedantic, but should I assume on Test Day that "changes in equilibria" are synonymous with changes in Q value for the equilibrium equation? Or just anything related to this specific topic on "what they mean when they say X" would be very helpful. Thanks
As I understand it, the addition of new solute that with an equilibria-involved component (CrO4 2-) alters the Q value/relative concentrations transiently, before this is counteracted per Le Chatelier.
I do not agree that "the equilibria shifted," as this may only occur upon changes in temperature. Maybe I'm being pedantic, but should I assume on Test Day that "changes in equilibria" are synonymous with changes in Q value for the equilibrium equation? Or just anything related to this specific topic on "what they mean when they say X" would be very helpful. Thanks
