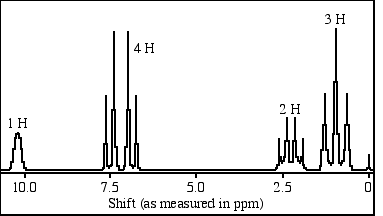
The H NMR peak at 7.37 ppm in Figure 1 can BEST be described as a:
a. doublet of doublets with a peak area ratio that is equal to 1 : 2 : 2 : 1
b. doublet of doublets with a peak area ratio that is equal to 1 : 1 : 1 : 1
c. quartet with a peak area ratio of 1 : 3 : 3 : 1
d. quartet with a peak area ratio of 1 : 2 : 2 : 1
Answer A is the best answer. The peak is not a quartet, because the baseline drops to zero in the middle, and the symmetry is not even throughout (i.e., the peaks are not evenly spaced). This eliminates choices C and D. The ratio of the peaks is not 1 : 1 : 1 : 1, so choice B can be eliminated. Perhaps it is not a perfect 1 : 2 : 2 : 1 ratio, but choice A is the best answer. The best answer is A
I'm a little confused as to why its not considered a quartet.

