Kaplan
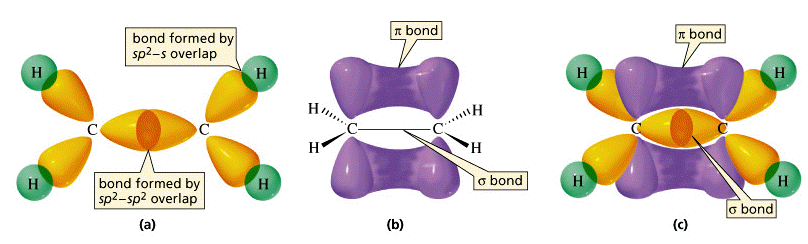
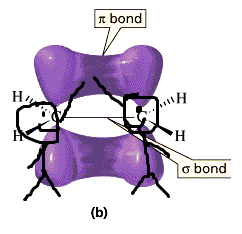
What's this about an electron being located above and below the nuclei?
A molecule is conjugated because it is able to delocalize pi electrons, because they are located above and below the nuclei, and can thus be delocalized to either side of the atom
What's this about an electron being located above and below the nuclei?