To remind us all re: MA.20....
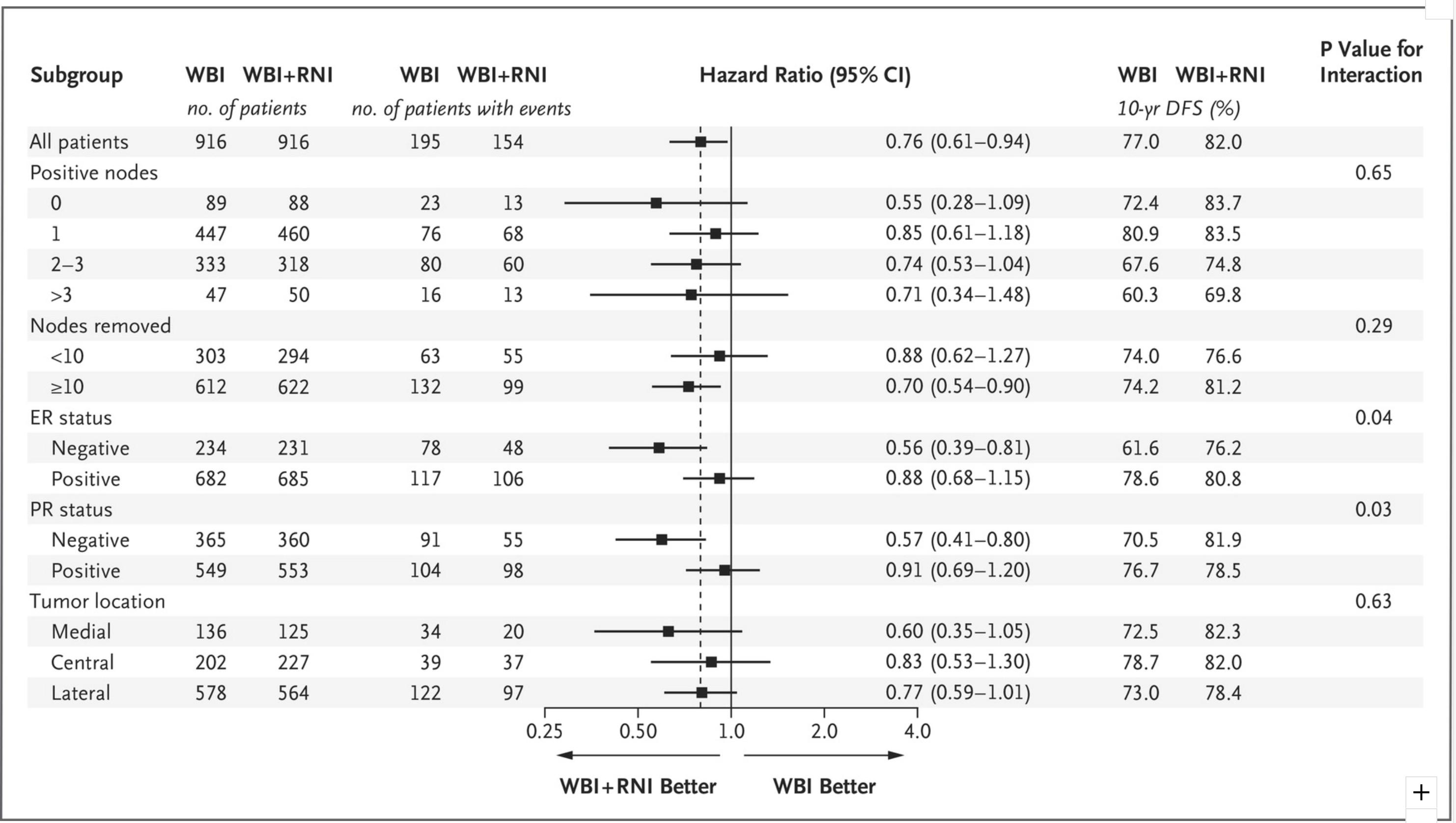
"The primary outcome was overall survival (82.3% vs 80.7%, p=0.06).
Secondary outcomes were disease-free survival (82.0% vs 77.0%, p=0.01),
isolated locoregional disease-free survival (95.2% vs 92.2%, p=0.009),
and distant disease-free survival (86.3% vs 82.4%, p=0.03)."
And...
"Patients in the nodal-irradiation group had higher rates of grade 2 or greater acute pneumonitis (1.2% vs. 0.2%, P=0.01),
and lymphedema (8.4% vs. 4.5%, P=0.001)."
And from discussion...
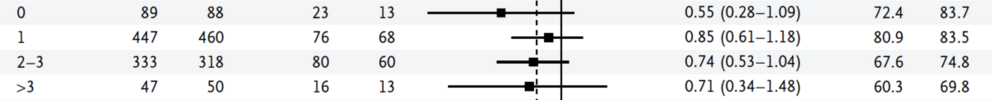
"Although subgroup analyses were prespecified, they were generally not adequately powered to assess the benefit of treatment in different subgroups. Furthermore, the P values of the subgroup analyses were not adjusted for multiple testing.
15 Patients with ER-negative or PR-negative tumors appeared to benefit more from regional nodal irradiation than those with ER-positive or PR-positive tumors. Although this effect was not observed in previous trials of postmastectomy radiotherapy,
16 it supports the hypothesis that further research on the molecular characterization of the primary tumor may identify patients who are more likely to benefit from regional nodal irradiation.
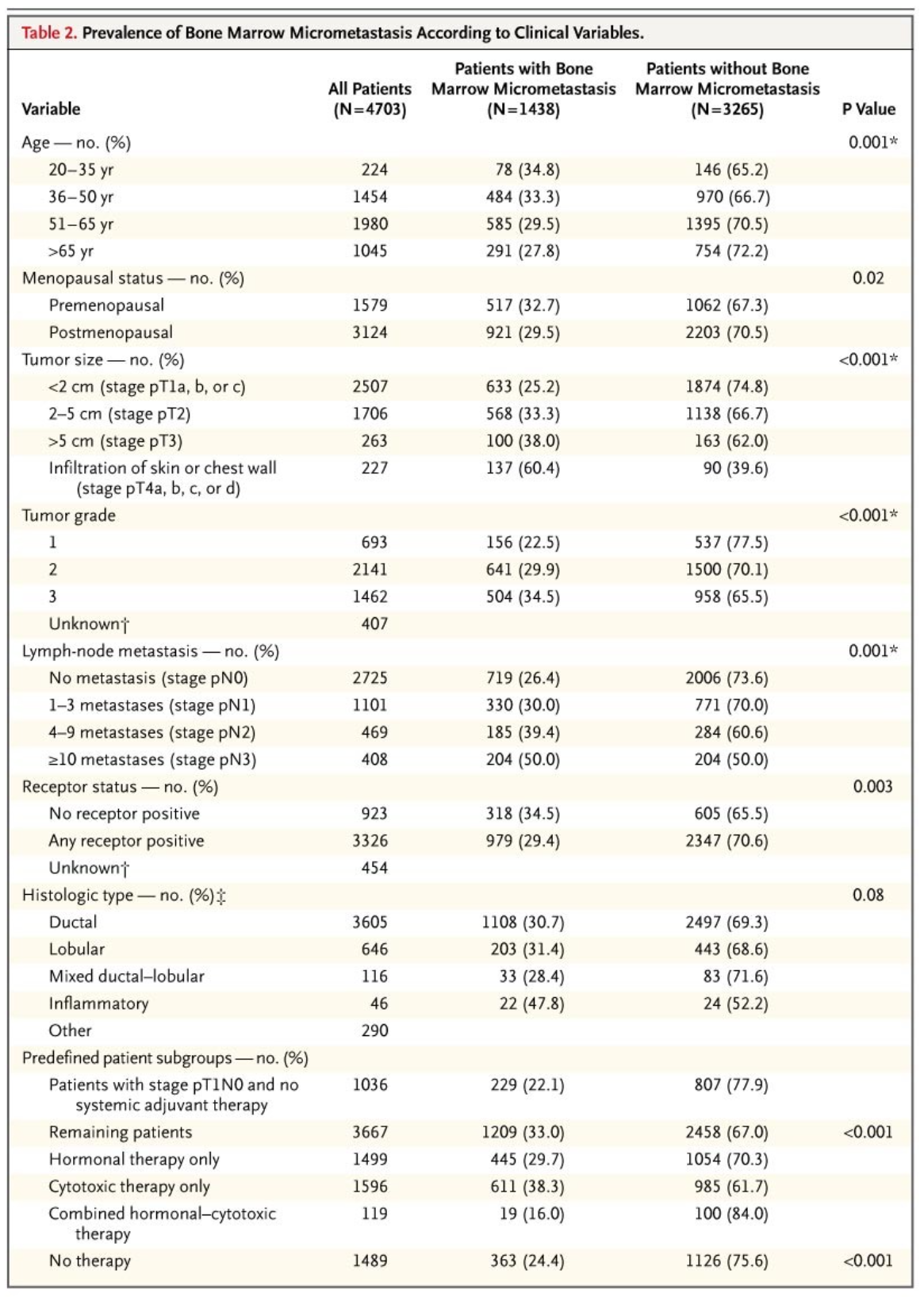
17,18 Since the number of node-negative patients in our trial was relatively small, the application of our results to node-negative patients is unclear. In addition, at the time of our study, the size of nodal metastasis was not routinely measured, so it is difficult to generalize our findings to patients with micrometastases."
Unknown: proportion of patients Her2+. Study was 2000-2007; from 2005 onward, patients could get herceptin.