- Joined
- Mar 23, 2014
- Messages
- 2,127
- Reaction score
- 2,276
Passage excerpt:
Genetics and cell biology based experiments link ARF1 to lipid droplet biogenesis. ARF1 function is regulated by the exchange of bound GDP for GTP via an exchange factor (e.g. GBF1) transitioning ARF1 from an inactive to an active conformation. This is then followed by hydrolysis of bound GTP to GDP transitioning ARF1 back to an inactive conformation. Due to its intrinsically low GTP hydrolysis rate, ARF1 requires an activating protein for GTP hydrolysis. In mammalian cells, three related activating proteins termed ARFGAP1-3 are linked to COPI vesicle formation through ARF of which ARFGAP1 is the prototypical protein.
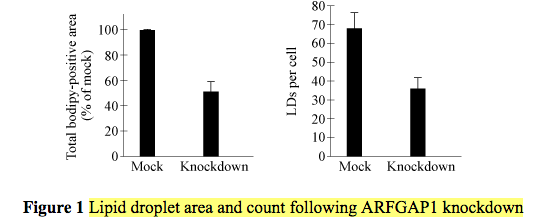
Then they knockdown ARFGAP1 and measure effect on the lipid droplets (vesicles).
My interpretation:
When ARFGAP1 is knocked out, lipid droplet counts per cell decreased. This seemed weird to me though, since ARFGAP1 inactivates ARF1 which helps makes the lipid droplets. I expected droplet counts to increase when ARFGAP1 (the inactivator) was knocked out. I guess I can't really assume a biological mechanism, though. Is it possible that ARFGAP1 inactivates ARF1 and in doing so somehow completes lipid droplet synthesis or release?
Source: http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0111309
Genetics and cell biology based experiments link ARF1 to lipid droplet biogenesis. ARF1 function is regulated by the exchange of bound GDP for GTP via an exchange factor (e.g. GBF1) transitioning ARF1 from an inactive to an active conformation. This is then followed by hydrolysis of bound GTP to GDP transitioning ARF1 back to an inactive conformation. Due to its intrinsically low GTP hydrolysis rate, ARF1 requires an activating protein for GTP hydrolysis. In mammalian cells, three related activating proteins termed ARFGAP1-3 are linked to COPI vesicle formation through ARF of which ARFGAP1 is the prototypical protein.
Then they knockdown ARFGAP1 and measure effect on the lipid droplets (vesicles).
My interpretation:
When ARFGAP1 is knocked out, lipid droplet counts per cell decreased. This seemed weird to me though, since ARFGAP1 inactivates ARF1 which helps makes the lipid droplets. I expected droplet counts to increase when ARFGAP1 (the inactivator) was knocked out. I guess I can't really assume a biological mechanism, though. Is it possible that ARFGAP1 inactivates ARF1 and in doing so somehow completes lipid droplet synthesis or release?
Source: http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0111309
