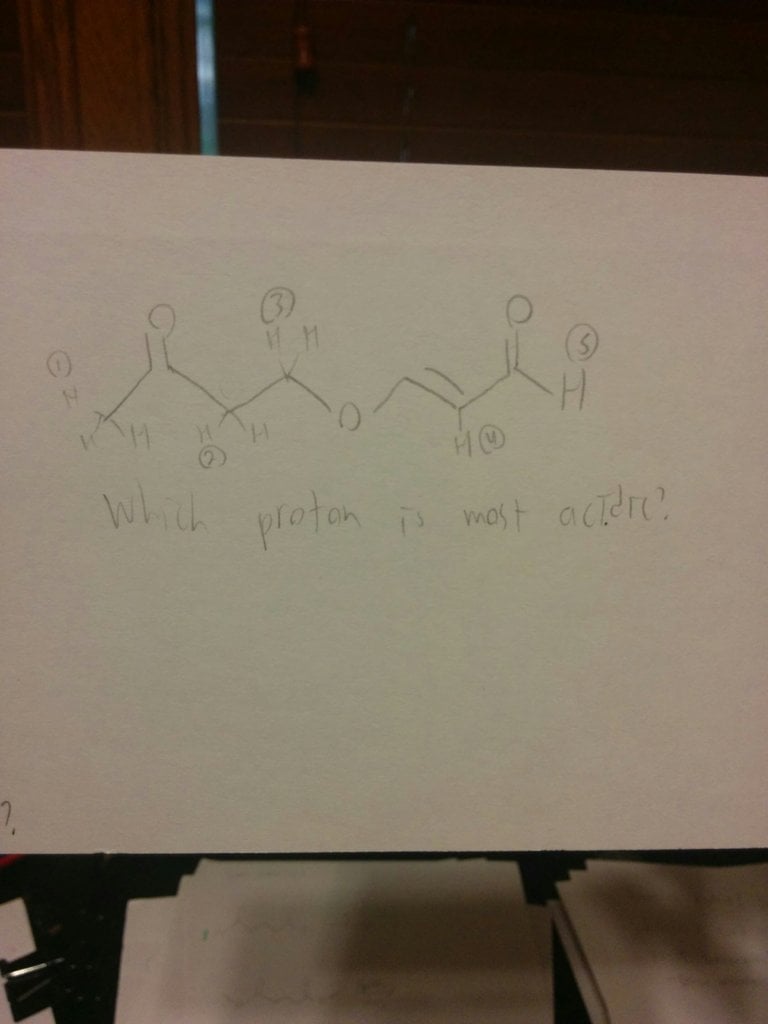
Can someone explain why it isn't the (2) H? I was always taught that a higher degree was more acidic when comparing two alpha h's. Like nah would turn the second one into enolate bc more substituted.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Destroyer q- which H is most acidic
- Thread starter 510586
- Start date
- Joined
- Mar 12, 2005
- Messages
- 4,786
- Reaction score
- 3,485
Can someone explain why it isn't the (2) H? I was always taught that a higher degree was more acidic when comparing two alpha h's. Like nah would turn the second one into enolate bc more substituted. View attachment 194867
You have brought up a very important concept. We have narrowed it down to either alpha proton 2 or alpha proton 1. Abstraction of proton 2 gives a very stabilized enolate.....more than the enolate in 1, as you say.
HOWEVER......the critical parameter here is kinetics.....the rate of abstraction. The proton on the outside is EASIER to remove. We now have a contest... Thermodynamics vs Kinetics. Kinetics wins ! Kinetics also wins out out in reactions such as Diels Alder. Thus....proton 1 is the most acidic.
If you want to see another example of this, go to any text book in organic chemistry and look up the Haloform reaction. You may have done the Iodoform reaction in lab. What is the first move ? We have 2 alpha protons.......The OUTERMOST proton is removed in preference over the innermost alpha proton. Kinetics wins.
Now,,,,,if a double alpha was present, such as that seen in reactions like AcetoAcetic ester synthesis, the double alpha would easily trump the outermost proton due to resonance stabilization.
This is not too tough.....You can do this !!!!
Dr. Romano
Oh yea the haloform reaction is a great example of this thank you! Makes it a lot easier to understand. Methyl ketones are better than others other than double alpha. So if we used the haloform reagent for a double alpha, it would form an enolate not a typical haloform reaction? ?You have brought up a very important concept. We have narrowed it down to either alpha proton 2 or alpha proton 1. Abstraction of proton 2 gives a very stabilized enolate.....more than the enolate in 1, as you say.
HOWEVER......the critical parameter here is kinetics.....the rate of abstraction. The proton on the outside is EASIER to remove. We now have a contest... Thermodynamics vs Kinetics. Kinetics wins ! Kinetics also wins out out in reactions such as Diels Alder. Thus....proton 1 is the most acidic.
If you want to see another example of this, go to any text book in organic chemistry and look up the Haloform reaction. You may have done the Iodoform reaction in lab. What is the first move ? We have 2 alpha protons.......The OUTERMOST proton is removed in preference over the innermost alpha proton. Kinetics wins.
Now,,,,,if a double alpha was present, such as that seen in reactions like AcetoAcetic ester synthesis, the double alpha would easily trump the outermost proton due to resonance stabilization.
This is not too tough.....You can do this !!!!
Dr. Romano
- Joined
- Mar 12, 2005
- Messages
- 4,786
- Reaction score
- 3,485
Oh yea the haloform reaction is a great example of this thank you! Makes it a lot easier to understand. Methyl ketones are better than others other than double alpha. So if we used the haloform reagent for a double alpha, it would form an enolate not a typical haloform reaction? ?
If a compound had a double alpha, it would NOT do the Haloform reaction. The Haloform reaction is reserved for methyl ketones or methyl carbinols. But, yes,,,,,you have the idea !!!!! Rock on !!!!!
Oh ok. Just to clarify, wouldn't a methyl ketone still be possible in a double alpha? Like two ketone's next to each other one being methyl? Thank you for helpingIf a compound had a double alpha, it would NOT do the Haloform reaction. The Haloform reaction is reserved for methyl ketones or methyl carbinols. But, yes,,,,,you have the idea !!!!! Rock on !!!!!
- Joined
- Mar 12, 2005
- Messages
- 4,786
- Reaction score
- 3,485
Oh ok. Just to clarify, wouldn't a methyl ketone still be possible in a double alpha? Like two ketone's next to each other one being methyl? Thank you for helping
Yes.....but it would not be a Haloform reaction. Once treated with base...we would set up the anion from the double alpha abstraction ...yes ? Once formed.....we can react this with a halide in an SN2 process,,,,,,,We see this in an AcetoAcetic Ester synthesis !!!
Oh ok so what you meant in the earlier post is the haloform is reserved for methyl ketones that are not part of a double alpha orientation. Got confused sorryYes.....but it would not be a Haloform reaction. Once treated with base...we would set up the anion from the double alpha abstraction ...yes ? Once formed.....we can react this with a halide in an SN2 process,,,,,,,We see this in an AcetoAcetic Ester synthesis !!!
Similar threads
- Question
- Replies
- 4
- Views
- 4K
- Replies
- 1
- Views
- 795
