- Joined
- Jun 18, 2013
- Messages
- 362
- Reaction score
- 52
TBR passage XIV, number 90:
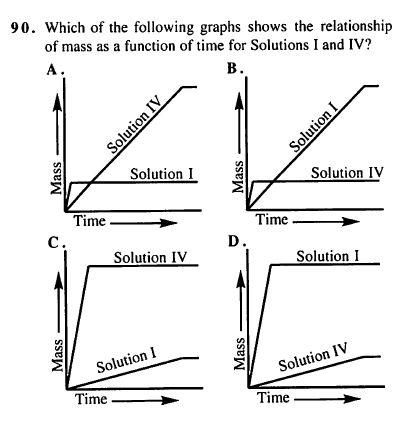
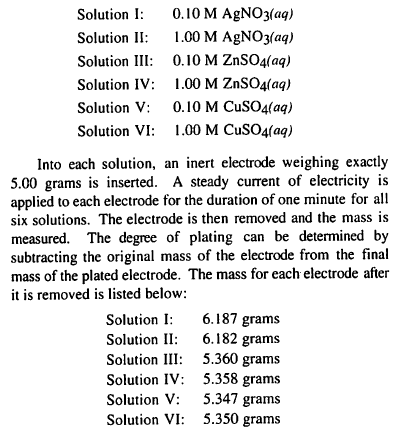
I thought it was D because it directly says the final masses in the passage ... I don't get it? Is the time scale going past the 1 min in the experiment or something? I know the solution IV has a higher molarity, but I thought that would just result in a longer time of increasing mass ... help?
I thought it was D because it directly says the final masses in the passage ... I don't get it? Is the time scale going past the 1 min in the experiment or something? I know the solution IV has a higher molarity, but I thought that would just result in a longer time of increasing mass ... help?
