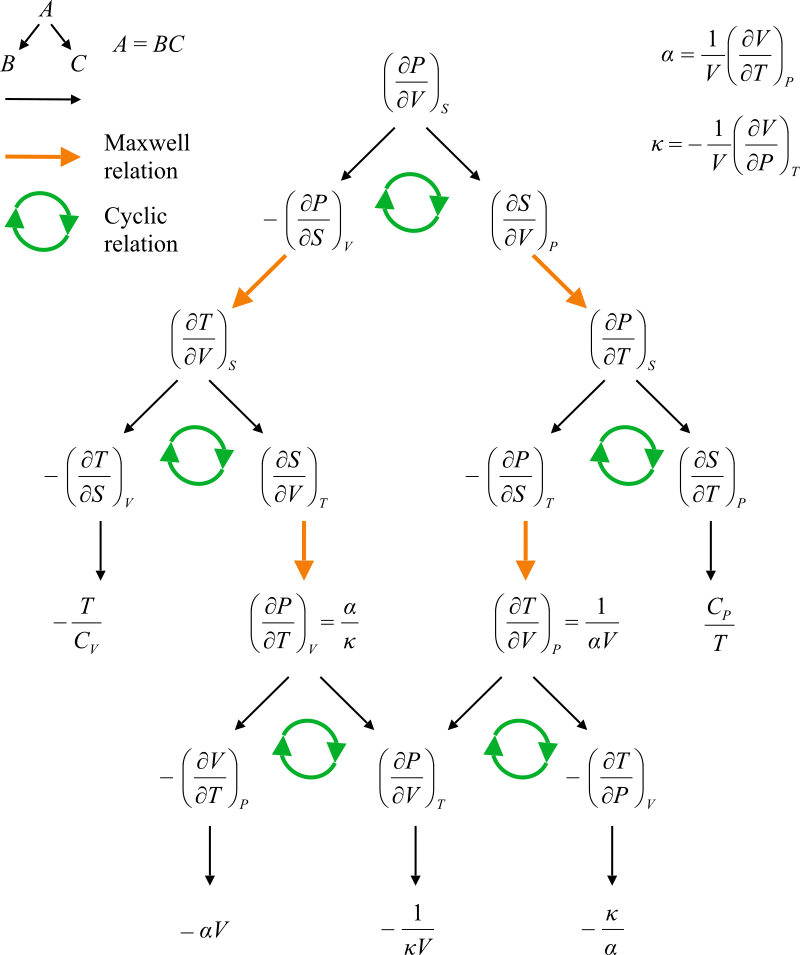
- Joined
- Mar 25, 2016
- Messages
- 88
- Reaction score
- 5
Hey SDN
I just got the syllabus for my gen chem course (not the honors version, still debating whether to sign up for it lol). We start off with thermochem: think Hess' Law, Enthalpy, q=mCdeltat, stuff like that.
Is this normal?
I just got the syllabus for my gen chem course (not the honors version, still debating whether to sign up for it lol). We start off with thermochem: think Hess' Law, Enthalpy, q=mCdeltat, stuff like that.
Is this normal?