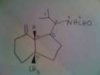
Not sure if the molecule is clear. The answer says there are 4 stereocenters but I'm not sure which ones. I labeled the possibilities with numbers. Please help!
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Help w/ stereocenters of this molecule
- Thread starter mflee
- Start date
- Joined
- Aug 22, 2008
- Messages
- 131
- Reaction score
- 0
Ok, so you need ones w/ 4 diff groups:
(sorry can't read your numbers)
The two that are attached to shaded bonds (showing they come out of the page) are definitely chiral carbons.
The "top" of the 5 carbon ring on the right (attached to one of the chiral centers) is also chiral. The carbon extending from that one which is attached to the isopropyl group and the NHCHO is your final chiral center.
How did I do it quickly? Any carbons with 2 hydrogen bonds (add them in in your head to the diagram!) is achiral. Any carbon with a double bond is achiral.
(sorry can't read your numbers)
The two that are attached to shaded bonds (showing they come out of the page) are definitely chiral carbons.
The "top" of the 5 carbon ring on the right (attached to one of the chiral centers) is also chiral. The carbon extending from that one which is attached to the isopropyl group and the NHCHO is your final chiral center.
How did I do it quickly? Any carbons with 2 hydrogen bonds (add them in in your head to the diagram!) is achiral. Any carbon with a double bond is achiral.
Last edited:
thanks! i read that the minimum number of different substituents needed is 3 (i guess this is for double bonds) even though it's usually 4. so i'm wondering why the N isn't a stereocenter too? or the carbonyl attached to the N?
- Joined
- Aug 22, 2008
- Messages
- 131
- Reaction score
- 0
Teeheehee, I love this part.
In order to have handedness, you have to have 2 sides or faces that are unique. An sp^2 hybridized atom (such as a carbon in a double bond) has a trigonal planar geometry, giving it two equal faces, i.e. achiral. Nitrogen has 3 bonds and a lone pair, so what geometry does it have?
In order to have handedness, you have to have 2 sides or faces that are unique. An sp^2 hybridized atom (such as a carbon in a double bond) has a trigonal planar geometry, giving it two equal faces, i.e. achiral. Nitrogen has 3 bonds and a lone pair, so what geometry does it have?
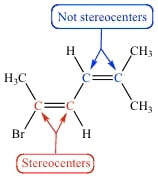
Right, but we're talking about being a stereocenter, not being chiral; an atom can be a stereocenter while being achiral, like the picture below. I'm not sure why the C=O or :N w/ 3 substituents aren't stereocenters 

- Joined
- May 17, 2010
- Messages
- 592
- Reaction score
- 42
also, wait...in the picture i just posted, why isn't the blue carbon on the left a stereocenter, while the red one on the right is? they both have 3 different substituents.
Where is that picture from?
- Joined
- May 17, 2010
- Messages
- 592
- Reaction score
- 42
Right, but we're talking about being a stereocenter, not being chiral; an atom can be a stereocenter while being achiral, like the picture below. I'm not sure why the C=O or :N w/ 3 substituents aren't stereocenters

I don't know if I can explain this all that well, but a stereocenter exists where, when you interchange two of its attached groups, the new molecule becomes a stereoisomer (stereoisomers differ in the way their atoms are oriented in space).
So, if you were to interchange the "red end" of the left-blue carbon's groups, nothing would change - at the other end of the left-blue carbon, it's symmetrical, and switching the left-blue C's groups wouldn't change any torsonial, nonbonded, or angle strain if the H and the red end switched spots.
However, if you switched the right-red carbon's H and blue end, then the whole molecule would be going from trans to cis and there would be competion for space (Br is huge), etc >> a stereoisomer would form! lol yay
thanks! i got that when i googled stereoisomer in google images. so with a double bond, does the change have to result in a cis-trans/trans-cis change? what i'm struggling with is that if you look at the left blue c, the "red end" and H are 2 different substituents
OHHHH I SEE! b/c the right side of the whole molecule is symmetrical, if you switch the H and "red end", you could still look at the molecule from the "behind it" and have the same thing as how we're looking at it now. so if the right end of the molecule wasn't symmetrical (i.e. was a methyl and ethyl group), and you switched the "red end" and H of the left blue C, you would get a stereoisomer right?
- Joined
- Jan 30, 2009
- Messages
- 4,218
- Reaction score
- 13
Sp3 nitrogen generally aren't considered chiral centers due to rapid inversion between the two forms. Can't remember what the term is for the phenomenon.
- Joined
- May 17, 2010
- Messages
- 592
- Reaction score
- 42
OHHHH I SEE! b/c the right side of the whole molecule is symmetrical, if you switch the H and "red end", you could still look at the molecule from the "behind it" and have the same thing as how we're looking at it now. so if the right end of the molecule wasn't symmetrical (i.e. was a methyl and ethyl group), and you switched the "red end" and H of the left blue C, you would get a stereoisomer right?
yep! switching the red C's groups (H and blue C) would change the way it's oriented in space - whatever that looks like (cis vs trans, or equatorial vs axial, something along those lines)
- Joined
- Mar 17, 2010
- Messages
- 242
- Reaction score
- 4
Sp3 nitrogen generally aren't considered chiral centers due to rapid inversion between the two forms. Can't remember what the term is for the phenomenon.
Yup - amines invert like umbrellas in a windstorm, they don't retain their chirality.
- Joined
- May 21, 2010
- Messages
- 68
- Reaction score
- 0
Looks like there's 4!
Similar threads
- Replies
- 4
- Views
- 4K
- Replies
- 1
- Views
- 2K
- Replies
- 1
- Views
- 484
- Replies
- 1
- Views
- 697