MCATExamkrackers
New Member
- Joined
- Jan 10, 2019
- Messages
- 7
- Reaction score
- 1
Hi, I’m Austin, one of the MCAT instructors at Examkrackers. For those of you gearing up to take the MCAT, I wanted to share some helpful points about one of the most commonly tested acids and bases questions on the exam. Talking about the equation has greatly benefitted our students in class, so I wanted to share our take on the equation that helps set and maintain our breathing rate, heart rate, and kidney function. If these words are starting to sound familiar, you’re probably thinking about the reaction catalyzed by carbonic anhydrase.
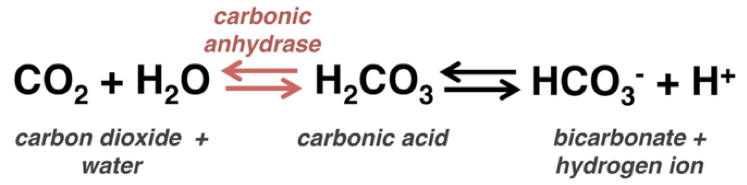
MCAT Think:You’ve just landed in Denver, Colorado and are hitting the ski slopes a few hours after you touch down. How does cutting tracks through fresh powder affect your blood pH?
Let’s say you start breathing faster because you score a lucky vacation to go skiing outside Denver (for MCAT completeness sake – Denver is about a mile above sea level, which means it has a lower density of oxygen compared to sea level. Your body senses this and makes you breathe faster to inspire more oxygen in the same amount of time).
Breathing faster means you’re going to exhale more carbon dioxide, pulling the equation to the left (aka Le Châtelier’s Principle). Pulling the equation to the left decreases the concentrations of bicarbonate and protons. Decreasing the concentration of protons increases the pH (remember pH = - log[H+], so the lesser the number of protons, the higher the pH), meaning that, in medical terms, your blood pH would increase and you’d become alkalotic.
If you’ve ever heard of altitude sickness, the reasoning is exactly the same as we just discussed, and can cause dizziness, shortness of breath, sleep problems, and loss of energy. As doctors, one way we can treat altitude sickness is to prescribe a medication called acetazolamide. Acetazolamide is a carbonic anhydrase inhibitor, so it prevents the conversion of bicarbonate and protons to carbon dioxide and water. This maintains the normal blood acidity, helping to alleviate the symptoms commonly associated with altitude sickness. Hopefully this explanation gives you a leg up when you see the carbonic anhydrase reaction on the exam!
MCAT Think:You’ve just landed in Denver, Colorado and are hitting the ski slopes a few hours after you touch down. How does cutting tracks through fresh powder affect your blood pH?
Let’s say you start breathing faster because you score a lucky vacation to go skiing outside Denver (for MCAT completeness sake – Denver is about a mile above sea level, which means it has a lower density of oxygen compared to sea level. Your body senses this and makes you breathe faster to inspire more oxygen in the same amount of time).
Breathing faster means you’re going to exhale more carbon dioxide, pulling the equation to the left (aka Le Châtelier’s Principle). Pulling the equation to the left decreases the concentrations of bicarbonate and protons. Decreasing the concentration of protons increases the pH (remember pH = - log[H+], so the lesser the number of protons, the higher the pH), meaning that, in medical terms, your blood pH would increase and you’d become alkalotic.
If you’ve ever heard of altitude sickness, the reasoning is exactly the same as we just discussed, and can cause dizziness, shortness of breath, sleep problems, and loss of energy. As doctors, one way we can treat altitude sickness is to prescribe a medication called acetazolamide. Acetazolamide is a carbonic anhydrase inhibitor, so it prevents the conversion of bicarbonate and protons to carbon dioxide and water. This maintains the normal blood acidity, helping to alleviate the symptoms commonly associated with altitude sickness. Hopefully this explanation gives you a leg up when you see the carbonic anhydrase reaction on the exam!
