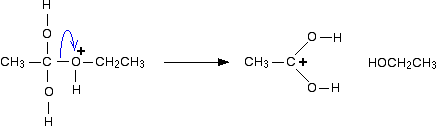
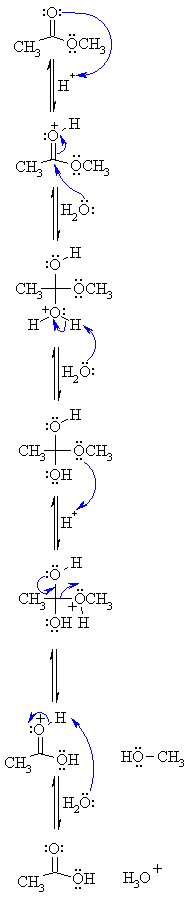
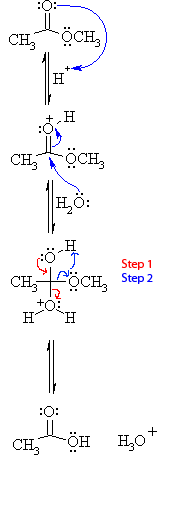
Ok, so I've seen a few FL question, where it asks: What would happen if you radioactively labeled the oxygen...blah blah..
If you radioactively-labeled the oxygen in the water nucleophile (e.g. O-18) in the following 3 reactions, would you see the labeled oxygen only in the resulting carboxylate product?
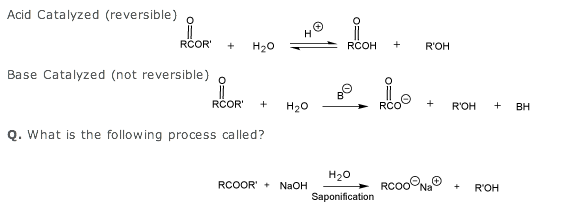
I think so b/c the OH group of the alcohol products are actually formed form the 'OR leaving group of the ester.
 ?
?
If you radioactively-labeled the oxygen in the water nucleophile (e.g. O-18) in the following 3 reactions, would you see the labeled oxygen only in the resulting carboxylate product?

I think so b/c the OH group of the alcohol products are actually formed form the 'OR leaving group of the ester.