- Joined
- Aug 26, 2014
- Messages
- 35
- Reaction score
- 6
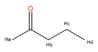
I attached an image for the nmr of 2-pentanone and I do not understand why there is a sextet. On my other attached image, Hc would be split by both Hd and Hb. I don't understand why this would be a sextet if it is being split by two unequivalent hydrogen groups within a 3 sigma bond distance. On the NMR, for Hc why do we not see a triplet of quartets, or a quartet of triplets? I'm probably missing something here. Some help would be very appreciated! Thank you