Two questions
1)I'm confused about the whole water trivia paragraph in TBR page 74. I understand that liq water is more dense than solid form. But I still don't understand the ice skating scenario. It says that warm water is at the BOTTOM of the lake. I read up online that warm water is LESS dense than cold. So, shouldn't the warm water (which is less dense) rise above the cold water (which is denser)?? Also, WHY is cold water more dense? You would think that cold water is closer to being a solid, which is less dense than liquid..
Also
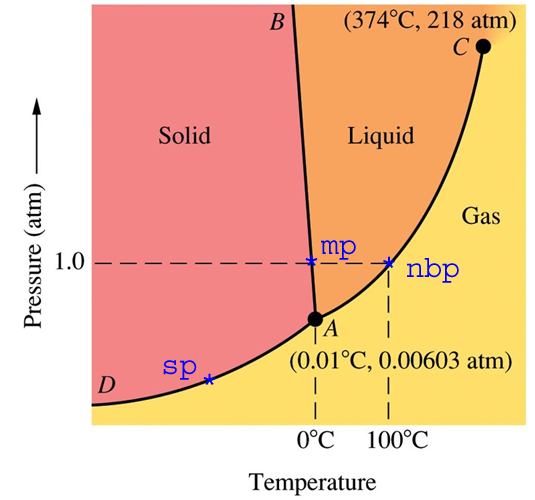
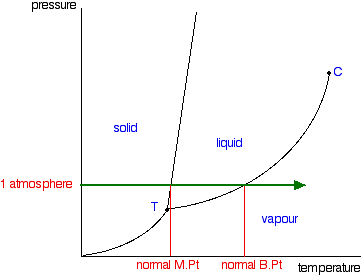
2) It says that isothermal increase in pressure results in liquid being denser than solid. Well, if I increased the pressure (and kept pressure constant) for another substance asides from water, doesn't an increase in Pressure decrease the Volume, and also make the liquid denser (see phase diagram)??
1)I'm confused about the whole water trivia paragraph in TBR page 74. I understand that liq water is more dense than solid form. But I still don't understand the ice skating scenario. It says that warm water is at the BOTTOM of the lake. I read up online that warm water is LESS dense than cold. So, shouldn't the warm water (which is less dense) rise above the cold water (which is denser)?? Also, WHY is cold water more dense? You would think that cold water is closer to being a solid, which is less dense than liquid..
Also
2) It says that isothermal increase in pressure results in liquid being denser than solid. Well, if I increased the pressure (and kept pressure constant) for another substance asides from water, doesn't an increase in Pressure decrease the Volume, and also make the liquid denser (see phase diagram)??
Last edited: