Someone posted something above re: MORE homogeneity, better control...
https://www.researchgate.net/public...ain_Metastases_Implications_for_Local_Control
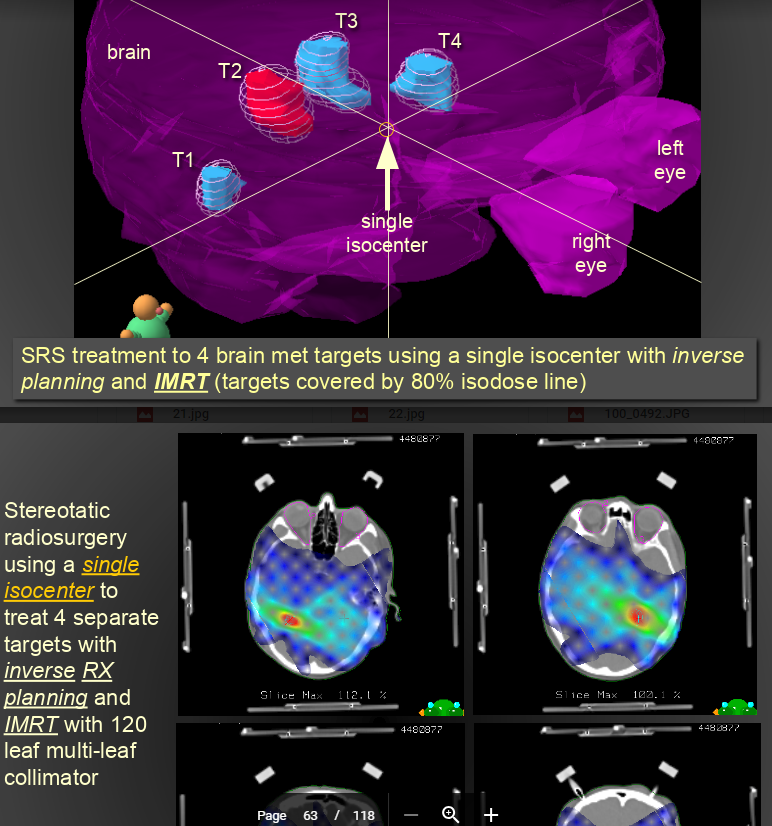
Of course, take w/ grain of salt. And, there have been some series in the AVM literature relating toxicities w/ IDLs/homogeneity/heterogeneity, "hot spots." Of course, back to semantics again, all these terms are mathematically interchangeable: homogeneity, heterogeneity, IDLs, "hot spot."
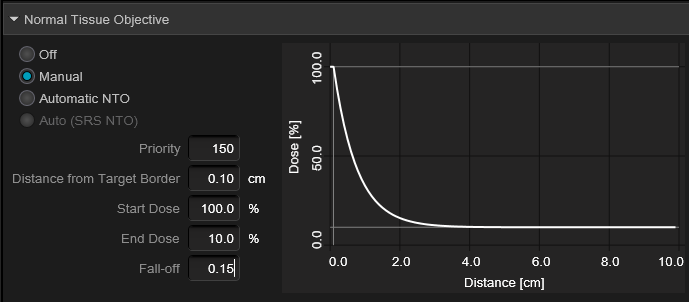
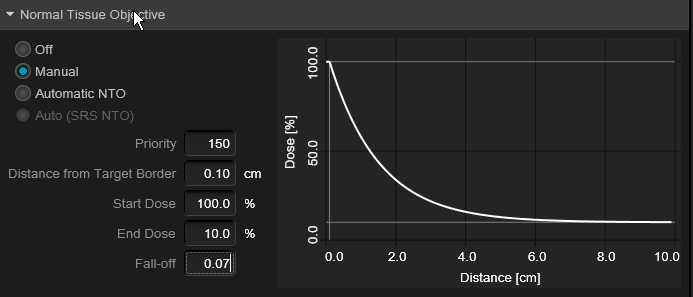
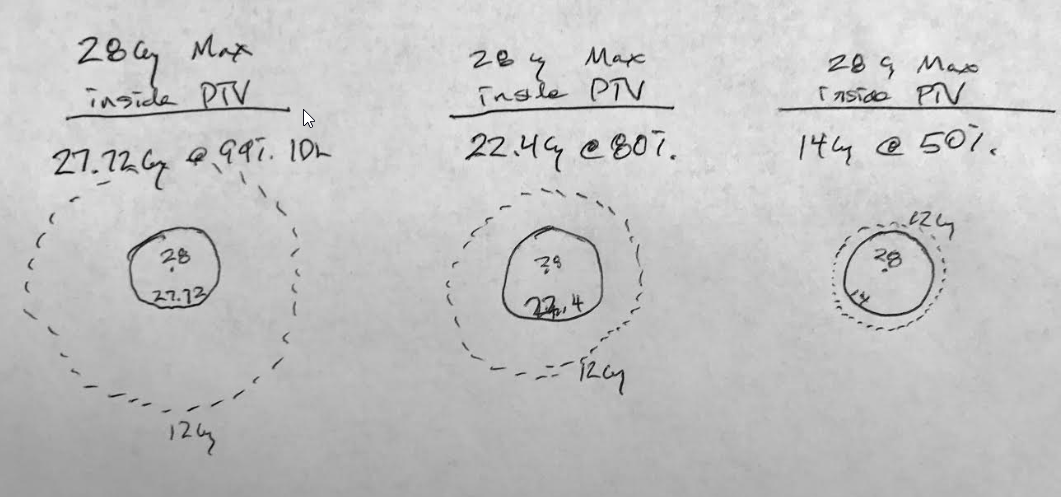
@emt409, let's consider just a solitary met, and per your paper "The prescription for all targets was standardized to 18 Gy" ...so with a 1 cc lesion, you would expect (and per your plot) a V12 of 4.5cc's. Assuming minimum lesional dose of 18 Gy, and that a typical plan for you would have ~150% "hot spot" (you said 130-170% somewhere), this would be akin to an Rx IDL of 67% ("normalizing" the 150% to 100%) and the V12 would be the 44% IDL. A 1cc lesion has about a 1.25 cm diameter; the 4.5 cc 44% IDL has a diameter of about 2.04 cm. This would mean the 44% IDL would be (2.04-1.25 divided by 2) 0.4 cm from the surface of the lesion (and the 50% IDL would be closer than that) implying a very rapid fall off from the 1cc target surface (a good plan). You can calc for other target cc's...
Target vol: 1cc, Distance of V12 from target surface: 0.40cm
Target volume: 2cc, Distance of V12 from target surface: 0.38cm
Target volume: 4cc, Distance of V12 from target surface: 0.37cm
Target volume: 8cc, Distance of V12 from target surface: 0.40cm
Target volume: 10cc, Distance of V12 from target surface: 0.41cm
Target volume: 15cc, Distance of V12 from target surface: 0.45cm
Target volume: 20cc, Distance of V12 from target surface: 0.48cm
Across target diameters of ~1-3 cm (target vol's of 1-20 cc), the V12 is changing linearly in relation to the target volume (obviously), but its distance from the target is sort of parabolic, sort of constant. What this implies to me is that in order to achieve keeping this falloff so remarkably consistent (~4-5 mm from target surface) no matter your lesion size, the planner is choosing a higher and higher "hot spot" as the lesion size goes up in size in order to make that happen. In other words, it seems the planner chooses (or allows for) more homogeneity for smaller lesions and less homogeneity for larger lesions. Of course this assumes equal minimal lesional doses across varying target volumes. And, if you're "choosing" less homogeneity for larger lesions to keep the V12 so tight, this means prescribing to lower IDLs for larger and larger lesions. Clearly well done plans on the larger lesions to keep that V12 so tight, but I suppose I choose lower (single shot) Rx doses for larger lesions versus keeping the Rx the same and prescribing to a lower and lower IDL as the lesion gets bigger and bigger. Or just hypofract the largers