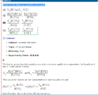
So I hit a question and i didn't quite know how to deal with it, nor did the explanation given when reviewing the exam give much insight.
The question is #25 on Kaplan FL9,
I've attached a print screen of the question and the explanation.
The basis for the answer i chose was just a guess and i moved on. I think in retrospect that after we get the Ka's that we must solve for something common to both sides of the equation. Since Bicarbonate is being used up as a reactant as it's being produced, it cannot be equal in both equations. This leaves H+, which i guess is a product of both reactions and would accumulate in the solution and reflect the pH. Thus, that would be the reason why we use that as a basis to set the two equations equal.
Am i on the right track here?
Much thanks,
-
The question is #25 on Kaplan FL9,
I've attached a print screen of the question and the explanation.
The basis for the answer i chose was just a guess and i moved on. I think in retrospect that after we get the Ka's that we must solve for something common to both sides of the equation. Since Bicarbonate is being used up as a reactant as it's being produced, it cannot be equal in both equations. This leaves H+, which i guess is a product of both reactions and would accumulate in the solution and reflect the pH. Thus, that would be the reason why we use that as a basis to set the two equations equal.
Am i on the right track here?
Much thanks,
-