- Joined
- Jun 30, 2005
- Messages
- 2,780
- Reaction score
- 2
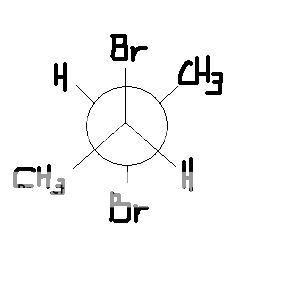
choose the true statment.
a) this is meso
b) this is (2S, 3S).
c) this is chiral
d) specific rotation is above 0 degree.
e) this is (2R,3R).
I think the answer key says A but I am not sure about this.
if this is meso, can someone teach me how to draw newman projection like this in a plane sheet of paper so I can tell whether it has internal symmetery or has S or R orientation?
I have tried many times and not sure which one is correct way of drawing among
......Br
...H-C-CH3
CH3-C-H
.......Br
and
......Br
CH3-C-H
CH3-C-H
.......Br
and
......CH3
...H-C-Br
CH3-C-H
.......Br
and so on.


