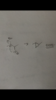Chapter 8 #14
Can someone explain why this reaction is SN1 instead of SN2?
The reagent is a strong base+nucleophile (OH-), and the halide is secondary. The product is inverted...so wouldn't this be the ideal SN2 condition?
Answer choice says B (SN1) because an inversion has occurred
Can someone explain why this reaction is SN1 instead of SN2?
The reagent is a strong base+nucleophile (OH-), and the halide is secondary. The product is inverted...so wouldn't this be the ideal SN2 condition?
Answer choice says B (SN1) because an inversion has occurred